Quadruple pulmonary vein stenting for post-ablation stenosis
Supported by the EuroIntervention Journal
Authors
Arkadiusz Pietrasik¹*, Karolina Jasińska-Gniadzik¹*, Rafał Maciąg², Monika Budnik¹, Aleksandra Gąsecka¹, Michał Sajdek², Marcin Grabowski¹, Janusz Kochman¹
- 1st Chair and Department of Cardiology, Medical University of Warsaw, Warsaw, Poland
- Second Department of Clinical Radiology, Medical University of Warsaw, Warsaw, Poland
* AP and KJG contributed equally to the article and share the first authorship.
Introduction
Pulmonary vein stenosis (PVS) is a complication of catheter ablation for atrial fibrillation (AF), occurring in 0.5-4 % of patients1, leading to morbidity and quality of life impairment.
While balloon angioplasty is often the initial approach, recurrent or resistant PVS may necessitate stenting2.
Case summary
A 64-year-old male patient with pulmonary hypertension and suspected iatrogenic PVS after pulmonary vein isolation due to AF was admitted for percutaneous angioplasty of pulmonary veins (PVs).
Several months after the index ablation procedure, patient reported gradually increasing dyspnea and fatigue (NYHA IV class), accompanied by paroxysmal cough with hemoptysis. The patient required home oxygen therapy and was repeatedly hospitalised.
A thoracic CT angiogram and transesophageal echocardiography (TEE) revealed significant stenosis involving all 4 PVs. Transthoracic echocardiography showed features of pulmonary hypertension, with systolic pulmonary artery pressure (SPAP) 70 mmHg. The patient was qualified by Heart Team for percutaneous treatment.
The procedure was performed via right femoral vein under TEE guidance. Using a BRK-1 needle, a transseptal puncture was performed. A JR 3.5 catheter was introduced into the left atrium through the Agilis NxT steerable sheath. After inserting the guidewire into each PV, intravascular ultrasound (IVUS) was performed, confirming the presence of a stenotic lesion and allowing measurement of reference vessel diameter (Figure 1 - A, C, E, G).
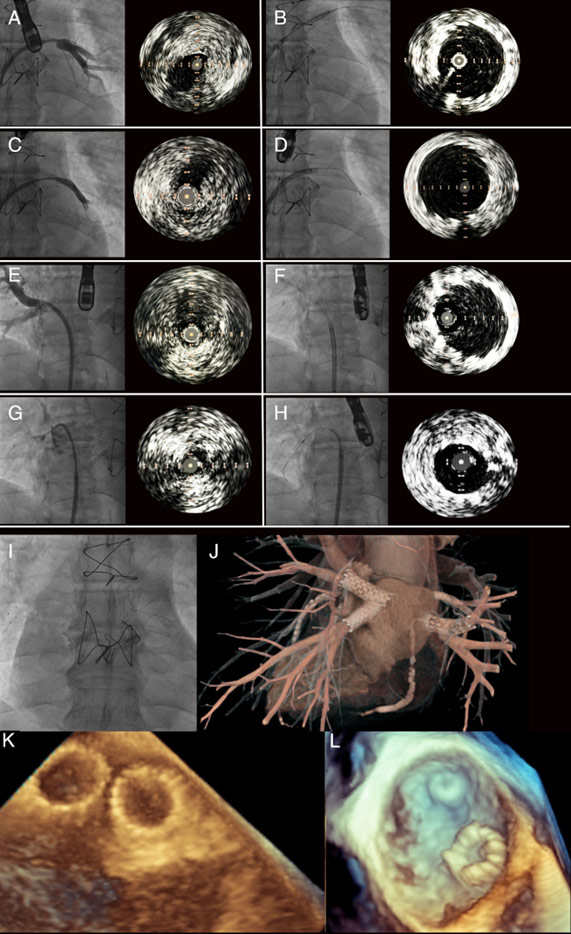
Figure 1. Pulmonary veins stenting. Angiography and IVUS of left superior PV before (A) and after (B) stenting. Angiography and IVUS of left inferior PV before (C) and after (D) stenting. Angiography and IVUS of right inferior PV before (E) and after (F) stenting. Angiography and IVUS of right superior PV before (G) and after (H) stenting. Fluoroscopic image (L) and 3D CT visualization (J) of all 4 stents. TEE image of stents in left PVs (K) and right PVs. CT – computed tomography, IVUS – intravascular ultrasound. PV pulmonary vein, TEE- transesophageal echocardiography.
After predilatation with a 9.0 x 40mm balloon catheter (12 atm), self-expandable stent was implanted in the left superior PV (Protégé 10 x 30 mm). Subsequently, after predilatation with a 9.0 x 40mm balloon catheter (12 atm), the self-expandable stent was implanted into the left inferior PV (Visi-Pro ev3 10 x 27 mm).
Next, a peripheral Cook ZF 10 x 40mm stent was implanted into the right inferior PV and postdilated with a 10 x 30mm balloon catheter (10 atm).
Finally, after predilatation with a 5.0 x 12mm balloon catheter (12 atm), a Nagomi Ultimaster 4.0 x 21 mm (16 atm) coronary stent was implanted into the right superior PV and postdilated with a non-compliant 5.0 x 12mm balloon (16 atm).
IVUS and TEE were used to guide stent deployment, ensuring optimal stent expansion and apposition without compromising flow into the left atrium (Figure 1 - B, D, F, H, K, L). Post-stenting CT showed correct position of stents in PVs (Figure 1 - J). Echocardiography demonstrated a decrease in SPAP to 30 mmHg.
The patient was discharged on post-procedural day 2. His symptoms markedly improved already a day following stenting, with resolution of dyspnea and cough, and cessation of home oxygen therapy after discharge.
A follow-up CT was recommended 6-9 months after the procedure.
Take-home messages
This case highlights the complex management of severe PVS following AF ablation. PV stenting might be required in case of extensive stenosis or chronic total occlusion. Self-expanding stents provide long-term patency in patients with PVS3.
Intravascular imaging, particularly IVUS, is critical to ensure proper stent deployment and optimize the effect.
Long-term data are needed to assess stent durability and restenosis rates. Further research should explore new devices and techniques for severe post-ablation PVS1. Moreover, vigilance should be maintained in post-ablation patients presenting with symptoms consistent PVS.
References
- Simard T, Sarma D, Miranda WR, Jain CC, Anderson JH, Collins JD, El Sabbagh A, Jhand A, Peikert T, Reeder GS, Munger TM, Packer DL, Holmes DR. Pathogenesis, evaluation, and management of pulmonary vein stenosis: JACC review topic of the week. J Am Coll Cardiol. 2023;81:2361–73.
- Agasthi P, Sridhara S, Rattanawong P, Venepally N, Chao C-J, Ashraf H, Pujari SH, Allam M, Almader-Douglas D, Alla Y, Kumar A, Mookadam F, Packer DL, Holmes DR Jr, Hagler DJ, Fortuin FD, Arsanjani R. Safety and efficacy of balloon angioplasty compared to stent-based-strategies with pulmonary vein stenosis: A systematic review and meta-analysis. World J Cardiol. 2023;15:64–75.
- Batool I, Khan MK, Zohaib M, Khan IA, Bukhari SAA, Shah S, Harmouch K, Hennawi HA, Klugherz B. Comparing patency of pulmonary Stent implantation and balloon angioplasty in pulmonary vein stenosis: A Systematic review and meta-analysis. Pulm Circ. 2025;15:e70036.
Conflicts of interest
The authors do not have any disclosures.





No comments yet!