26 results
The importance of PPM in younger patients in TAVI valve choice
08 Dec 2025
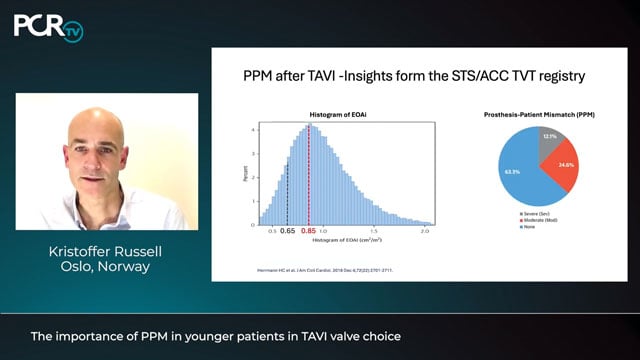
Kristoffer Russell discusses prosthesis–patient mismatch (PPM), which can increase mortality, heart failure hospitalisations, and reduce functional recovery, especially in younger patients. While less common after TAVI than surgical valve replacement, studies show up to 30% of younger, low-risk TAVI patients still experience moderate or severe PPM,...
TAVI session from PCR London Valves 2025 : Abbott
26 Nov 2025
Watch these recordings from PCR London Valves 2025 on TAVI.
Mitral session from PCR London Valves 2025: Abbott
26 Nov 2025
Watch these recordings from PCR London Valves 2025 and EuroPCR 2025 on Mitral.
Tricuspid Valve sessions from PCR London Valves 2025: Abbott
26 Nov 2025
Watch these recordings from PCR London Valves 2025 and EuroPCR 2025 on tricuspid valve.
Targeting inflammation in AMI: a debate - Video replay
16 Aug 2024
Watch this session to explore inflammation's role in AMI, including IL-6's significance and strategies for targeting inflammation.
CoreValve™ and Evolut™ TAVI systems
13 May 2022
Sponsored by Medtronic
Bifurcation Lesions Sessions
17 Feb 2022
Watch the recordings of these sessions on PCR in patients with complex lesions ACS and HBR and complex Bifurcation PCI: What to avoid?
Medtronic TAVI Sessions from PCR London Valves 2021
10 Dec 2021
Watch these recordings from PCR London Valves on TAVI.

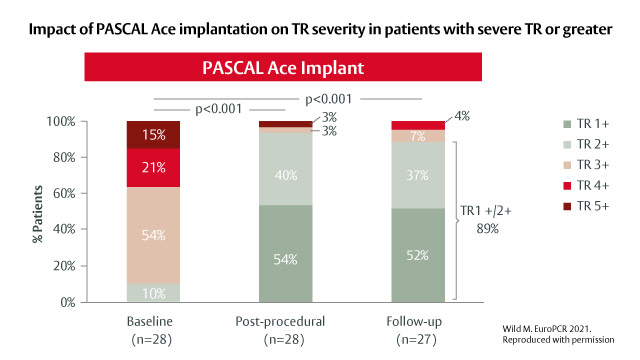
Real-world outcomes with the PASCAL Platform in TR
03 Dec 2021
The PASCAL platform, comprising the PASCAL and PASCAL Ace implants, received its CE mark for TR in May 2020 and has been shown to be an effective and safe treatment option for patients with TR.3,4,6 Professor Jörg Hausleiter and Dr Mirjam Wild now describe their single-centre experience...
TAVI sessions from PCR London Valves 2021: Edwards Lifesciences
21 May 2021
Watch the TAVI Edwards Benchmark Symposium