Restriction in supra-annular transcatheter heart valve leaflet mobility during coronary access after valve-in-valve TAVI
Supported by the EuroIntervention Journal
Authors
Arif A Khokhar1,2, Adriana Zlahoda-Huzior2,3, Francesco Giannini4, Dariusz Dudek4,5
Case summary
Coronary access after transcatheter aortic valve implantation (TAVI) can be challenging or unfeasible due to interactions between coronary catheters and elements of the transcatheter heart valve (THV) frames1-3. Catheters may also interact with THV leaflets, which, in the extreme case of leaflet pinning, may lead to acute haemodynamic compromise. However, to date, the interaction between THV leaflets and catheters during coronary cannulation has not been visualized or evaluated.
We developed an ex vivo computed-tomography-derived patient-specific anatomical model to simulate coronary cannulation procedures. (Supplementary Figure 1A) The model was connected to a pulsatile flow simulator to replicate in vivo conditions. Two supra-annular valves, Evolut (Medtronic, Minneapolis MN, USA) and ACURATE neo2 (Boston Scientific, Marlborough, MA, USA) were selected for evaluation due to the higher placement of their leaflets, which may increase the likelihood for catheter-leaflet interactions.
A 26 mm Evolut and Size S ACURATE neo2 were implanted inside a Carpentier-Edwards Perimount 25 mm (Edwards Lifesciences, USA) surgical bioprosthesis to replicate coronary access after valve-in-valve TAVI. (Supplementary Figure 1B).
Expert operators cannulated the coronary ostia using different catheters under fluoroscopic guidance. Interactions between the catheters and THV leaflets were visualized using an internally mounted borescope camera. (Supplementary Figure 1C)
Our images demonstrate how coronary catheters can interact with the leaflets of both supra-annular THVs (Figure 1). The degree of restriction in leaflet mobility varied from mild systolic leaflet restriction to more significant leaflet pinning throughout the cardiac cycle. The degree of leaflet restriction was dependent on the choice of catheter and the cannulation technique. Leaflet pinning was more frequently observed with the use of the supportive Amplatz Left 1 catheter or if an Extra Backup catheter was pushed down to assume a more supportive position (Supplementary Videos 1-2). These interactions were observed with both commissural alignment and misalignment of the transcatheter heart valves.
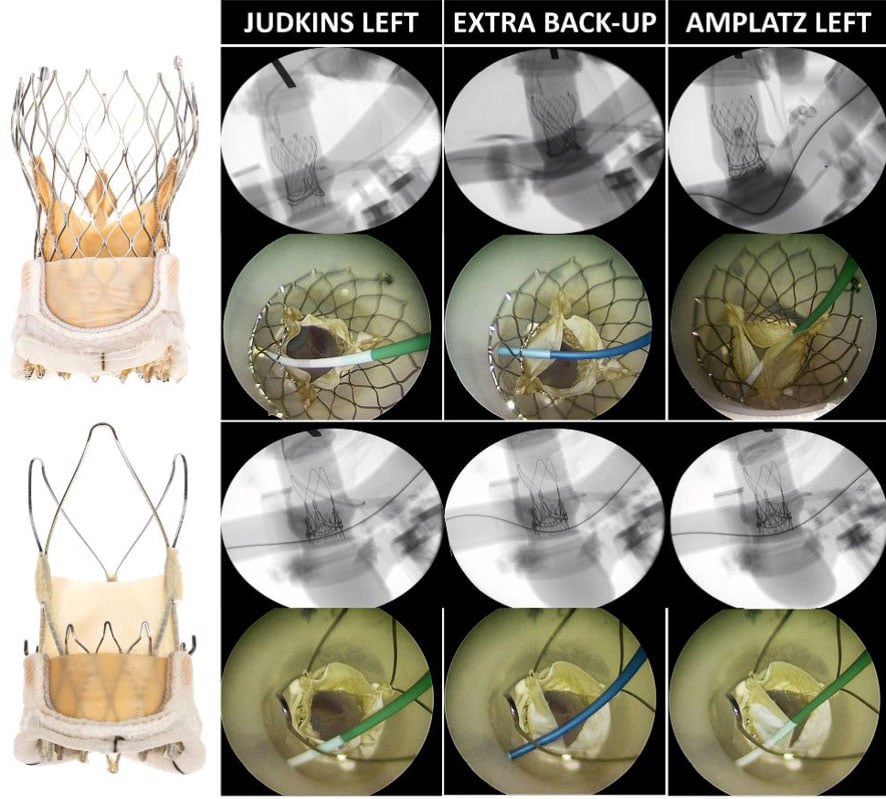
Figure 1: Visualizing interaction between Evolut and ACURATE neo2 transcatheter heart valve leaflets and catheters during coronary access after valve-in-valve TAVI
Different degrees of leaflet interactions were observed depending on the choice of catheter used for coronary cannulation. Use of the Judkins Left catheter resulted in minimal restriction of leaflet mobility. In contrast use of the Extra Back-Up and Amplatz Left 1 guiding catheters caused increased restriction in leaflet mobility (red arrow) and even leaflet pinning.
The hemodynamic consequences of these restrictions in leaflet mobility are unknown. Mild leaflet interaction or restriction that affects only a single leaflet during systole would likely have no significant impact. In contrast, complete pinning of one or more leaflets could result in acute aortic insufficiency and subsequent haemodynamic compromise.
Operators should therefore be aware of this phenomenon, particularly when using supportive catheters during long complex PCI procedures in patients who underwent TAVI.
Changing the type or position of the guiding catheter or utilising an external cannulation technique which bypasses the valve frame can minimise or avoid interactions with THV leaflets (Supplementary Figure 2) (Supplementary Videos 3-4).
These catheter-leaflet interactions were only evaluated for two specific self-expandable supra-annular THVs. However, it is likely that these interactions can occur with other THVs, particularly in patients with low-lying coronary arteries or following higher valve implantations.
Further testing in different anatomical settings using different THVs should be combined with hydrodynamic testing (transvalvular leakage, transvalvular gradients, and both effective and geometric orifice areas) to further evaluate the potential impact of these catheter-leaflet interactions.
Our work highlights how patient-specific ex vivo simulations offer physicians the opportunity to observe and evaluate the potential in vivo impact of different techniques particularly for complex structural heart interventions.
Supplementary Material
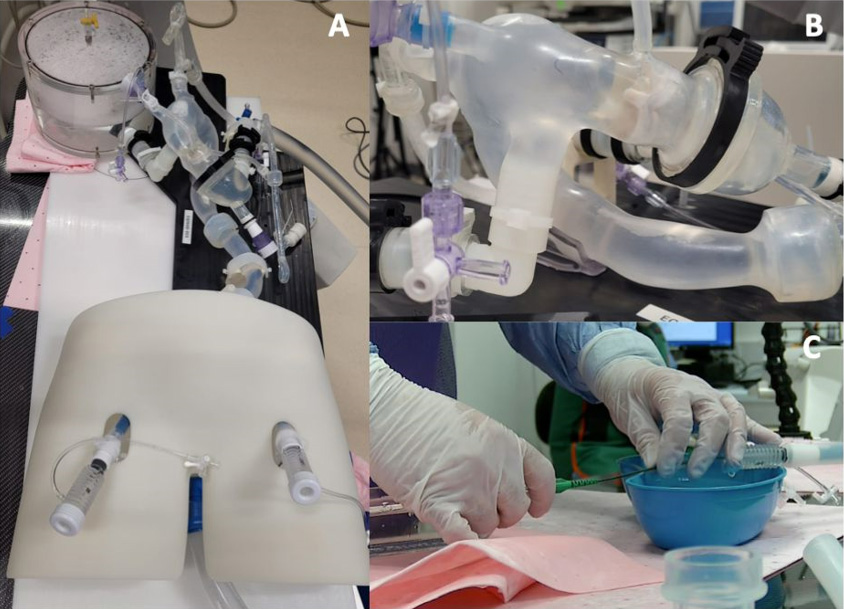
Supplementary Figure 1 : Patient-specific pulsatile flow simulator
(A) Ex vivo cannulations were performed using a patient-specific pulsatile flow model based on computed tomography data with (B) real transcatheter and surgical valves implanted inside a surgical bioprosthesis to replicate in vivo conditions. (C) Operators performed cannulation procedures under real catheterization laboratory conditions.
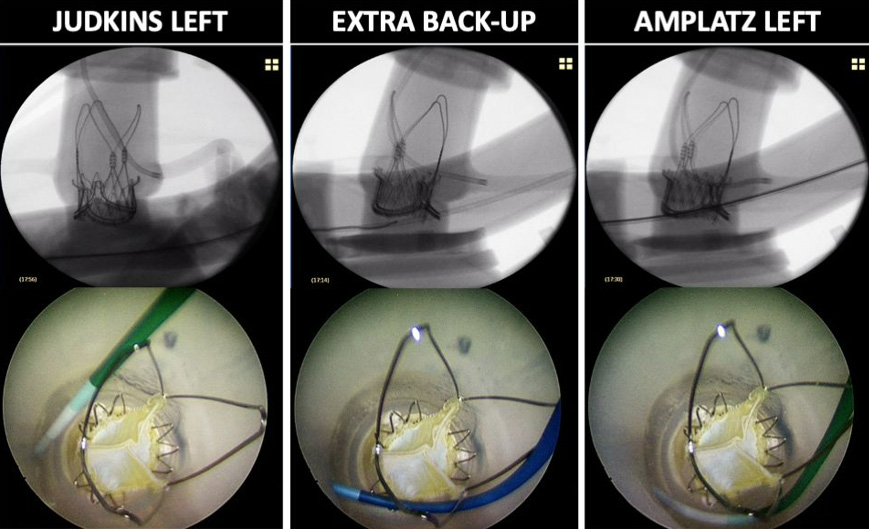
Supplementary Figure 2: External cannulation approaches with the ACURATE neo2
The open-celled stabilization arches of the ACURATE neo2 valve frame, allowed for an external cannulation approach, whereby the valve frame was bypassed using either the JL, EBU and AL guiding catheters to avoid any interaction with the valve leaflets.
Video 1: Pinning of Evolut valve leaflets by Amplatz Left 1 guiding catheter
Video 2: Pinning of ACURATE neo2 valve leaflets by Amplatz Left 1 guiding catheter
Video 3: Impact of catheter positioning on leaflet mobility with ACURATE neo2
Video 4: External cannulation approaches with the ACURATE neo2
Conflict of interest statement
We wish to declare that the equipment and materials for this study were provided and partially funded by a research grant from Boston Scientific.
We confirm that Boston Scientific had no input into the planning, design, and conducting of this study.
We additionally wish to disclose that DD is on the advisory board for Boston Scientific.
References
- Barbanti M., Costa G., Picci A., et al. Coronary Cannulation After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2020;13(21):2542–55. Doi: 10.1016/j.jcin.2020.07.006.
- Tarantini G., Nai Fovino L., Scotti A., et al. Coronary Access After Transcatheter Aortic Valve Replacement With Commissural Alignment: The ALIGN-ACCESS Study. Circ Cardiovasc Interv 2022;15(2):e011045. Doi: 10.1161/CIRCINTERVENTIONS.121.011045.
- Valvo R., Costa G., Tamburino C., Barbanti M. Coronary artery cannulation after transcatheter aortic valve implantation. EuroIntervention 2021;17(10):835–47. Doi: 10.4244/eij-d-21-00158.
Affiliations
- Imperial College Healthcare NHS Trust, London, UK
- Digital Innovations & Robotics Hub, Krakow, Poland
- Department of measurement and electronics, AGH University of Science and Technology, Krakow, Poland
- Interventional cardiology unit, GVM Care & Research, Maria Cecilia Hospital, Cotignola, Italy
- Institute of Cardiology, Jagiellonian University Medical College, Krakow, Poland






No comments yet!