05 May 2020
Portico with FlexNav TAVI system: enhancing innovative technology to optimize patient outcomes and physician experience
Sponsored by Abbott
Real-world case report illustrating the benefits of the Portico with FlexNav TAVI system.
Achieving accurate valve deployment in complex anatomies
By Giulia Costa, MD, and Lars Søndergaard, MD, DMSC
The Portico valve (Abbott) is a transcatheter, self-expanding, resheathable device with intra-annular positioning of the bovine leaflets, which has proven safety and performance in several studies.1,2 The major advantages of this device include its low profile and flexibility, making it easier to overcome potential challenges related to tortuous and calcified vessels, as well as horizontal aortic anatomy. The next-generation delivery system (FlexNav, Abbott) recently took its first step into the clinical arena, offering additional improvements in deliverability and accurate valve deployment in complex anatomies.
Patient presentation
An 81-year-old woman was referred for evaluation of a severe symptomatic aortic stenosis that was detected several months ago but her symptoms rapidly progressed. The patient reported exertional dyspnea (New York Heart Association [NYHA] class III) that limited daily activities that she had previously undertaken without problems. Her clinical history included systemic hypertension, chronic obstructive pulmonary disease, recurrent pyelonephritis, and previous deep vein thrombosis and stroke. Moreover, the patient had undergone left mastectomy and radiation therapy for breast cancer and subsequent reoperation for relapse.
Her physical examination revealed obesity (body mass index, 31.1 kg/m²) but no signs of cardiac decompensation or renal impairment. An electro-cardiogram (ECG) showed normal sinus rhythm.
A transthoracic echocardiogram confirmed the diagnosis of severe calcific aortic stenosis (peak gradient, 95 mm Hg; mean gradient, 64 mm Hg; aortic valve area, 0.6 cm²) with preserved left ventricular ejection fraction but concentric left ventricular hypertrophy. The coronary arteries showed mild atherosclerotic disease without obstructive lesions.
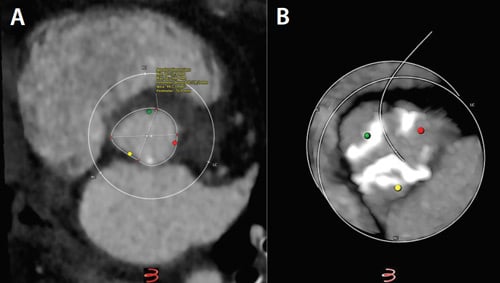
Figure 1. Aortic annulus dimensions (A) and “hockey-puck” view of valvular calcifications (B).
Treatment selection
The case was discussed in the multidisciplinary heart team meeting. Taking into consideration the overall risk profile (age, obesity, lung disease, previous thoracic radiation therapy), the patient was scheduled for transcatheter aortic valve implantation (TAVI).
The preprocedural CT scan showed an elliptical aortic annulus with a perimeter-derived diameter of 24.2 mm, valvular calcifications (Figure 1), and horizontal aortic angulation (Figure 2). The abdominal aorta and aortic arch were calcified, but the iliofemoral vessels were otherwise deemed suitable for a transfemoral approach (Figure 3).
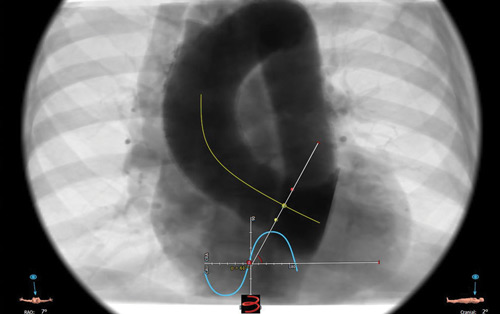
Figure 2. Aortic root angulation.
Due to the calcified aortic arch and horizontal aorta, the flexible Portico transcatheter heart valve (THV) was chosen and delivery was planned with the FlexNav system.
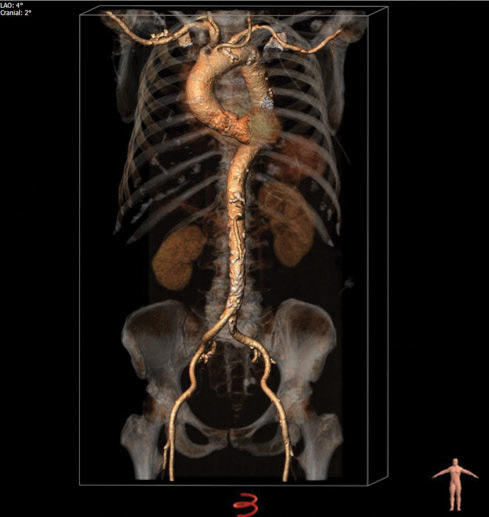
Figure 3. A three-dimensional volume-rendering reconstruction of the aorta and iliofemoral vessels.
Procedure description and results
The procedure was performed using local anesthesia and without sedation. The right femoral artery was chosen as the access route for the THV system. After establishing vascular access in the common femoral artery, a 14-F sheath was inserted over a stiff guidewire. The aortic valve was then crossed with an Amplatz left 2 catheter and a straight standard guidewire. Next, a preshaped stiff guidewire (Safari2, Boston Scientific Corporation) was placed in the left ventricle and predilatation was performed with a 22-mm True balloon (BD Interventional). Subsequently, a 27-mm Portico THV was loaded in the FlexNav delivery system, the 14-F sheath was removed, and the FlexNav delivery system was introduced using the integrated sheath. The flexibility of the delivery system allowed safe passing of the calcified aortic arch, as well as coaxial alignment in the aortic annulus. The Portico THV was successfully deployed without the need for pacing or repositioning.
The final aortogram showed only trace paravalvular leak (Figure 4).
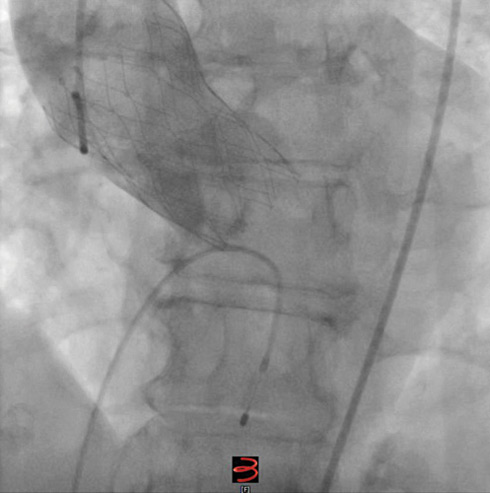
Figure 4. Final aortogram after implantation of the Portico THV.
The ipsilateral arterial access site was successfully closed with a Manta device (Teleflex). An ECG revealed unchanged normal sinus rhythm, and the temporary pacing lead was therefore removed at the end of the procedure. No procedural complications occurred, and the total procedural time was 90 minutes, including 45 minutes of skin-to-skin time.
The patient was discharged to home 48 hours after the procedure. A predischarge echocardiogram showed favorable prosthesis performance (mean gradient, 6 mm Hg; effective orifice area, 2.5 cm²) and absence of paravalvular leakage.
At 30-day follow-up, the patient reported a marked improvement in clinical symptoms (NYHA class I), with progressive resumption of normal everyday activities.
Discussion
TAVI is currently playing an increasing role in the treatment of symptomatic severe aortic stenosis. During the last decade, the technological evolution and increased learning experience has facilitated streamlining the procedures and simplifying the overall clinical course.
This even applies to high-risk patients with diseased peripheral vessels, horizontal aorta, and aortic annulus with unfavorable anatomy. Particularly in these often elderly patients with several comorbidities, it is mandatory to avoid complications that may not only prolong the hospital stay but also trigger a vicious cycle of clinical deterioration. Historically, vascular complications and bleeding have confounded the TAVI journey, leading to increased morbidity and mortality.3 The presence of a horizontal aorta represents a challenge for accurate THV placement, especially with less flexible systems.4 Finally, the presence of an elliptic aortic annulus may represent an additional challenge in the setting of self-expandable heart valves.5
This high-risk case demonstrates how the development of a new-generation THV system with a low insertion profile and high flexibility allows a simple and safe procedure with accurate THV placement, even in cases with challenging anatomic features. Thus, the Portico THV system allows passage even through severely tortuous and calcified peripheral vessels and overcomes the challenges of unfavorable aortic root angulations. The next-generation FlexNav delivery system promises further enhancement of such features, offering an integrated sheath and a hydrophilic coating. The outcomes of the ongoing FlexNav European Union CE Mark study (NCT03724812) and FlexNav arm of the Portico United States investigational device exemption trial (NCT02000115) will provide information about the safety and efficacy of the new-generation Portico delivery system in larger patient cohorts.
References
- Søndergaard L, Rodés-Cabau J, Hans-Peter Linke A, et al. Transcatheter aortic valve replacement with a repositionable self-expanding prosthesis: the PORTICO-I trial 1-year outcomes. J Am Coll Cardiol. 2018;72(23 Pt A):2859-2867.
- Linke A, Holzhey D, Möllmann H, et al. Treatment of aortic stenosis with a self-expanding, resheathable transcatheter valve: one-year results of the international multicenter Portico transcatheter aortic valve implantation system study. Circ Cardiovasc Interv. 2018;11:e005206.
- Okuyama K, Jilaihawi H, Abramowitz Y, et al. The clinical impact of vascular complications as defined by VARC-1 vs. VARC-2 in patients following transcatheter aortic valve implantation. EuroIntervention. 2016;12:e636-e642.
- Abramowitz Y, Maeno Y, Chakravarty T, et al. Aortic angulation attenuates procedural success following self-expandable but not balloon-expandable TAVR. JACC Cardiovasc Imaging. 2016;9:964-972.
- Maeno Y, Abramowitz Y, Yoon SH, et al. Transcatheter aortic valve replacement with different valve types in elliptic aortic annuli. Circ J. 2017;81:1036-1042.
Giulia Costa, MD
Department of Cardiology, Rigshospitalet
University of Copenhagen
Copenhagen, Denmark
Disclosures: None.
Lars Søndergaard, MD, DMSc
Department of Cardiology, Rigshospitalet
University of Copenhagen
Copenhagen, Denmark
Disclosures: Consultant fees and institutional research grants from Abbott.
Portico With FlexNav TAVI System: Enhancing Innovative Technology to Optimize Patient Outcomes and Physician Experience
By Mike Morrissey
The original Portico transcatheter aortic valve implantation (TAVI) system (Abbott) comprises a transcatheter valve and a delivery system used to navigate and deploy the valve into position at the native aortic annulus. Throughout the initial clinical experience with the Portico system, several aspects were identified as major strengths. These included the low profile and overall flexibility of the delivery system; ease of tracking the system over the aortic arch; intra-annular valve positioning for hemodynamic stability during deployment; recapturability, repositionability, and retrievability of the valve; and large-cell geometry for easy coronary access postimplantation.
Over time, additional areas for improvement were identified as physicians expressed a need for sheathless vascular access, valve placement accuracy, and overall improvements in ease of use.
Latest-generation TAVI system
As a response to these customer requests, the FlexNav delivery system (Abbott; Figure 1) was developed to address improvement needs while maintaining the positive performance aspects of the original Portico system (see the Technology Design Goals Met By the FlexNav Delivery System sidebar). The FlexNav delivery system incorporates a stability layer to the catheter, reducing the amount of manipulation required at the access site and providing predictable, accurate, and stable implantation of the valve at the annulus. The addition of an integrated sheath onto the catheter allows easy, sheathless access into the vasculature and maintains a low insertion profile, allowing access into vessels as small as 5 mm for the small system and 5.5 mm for the large system.
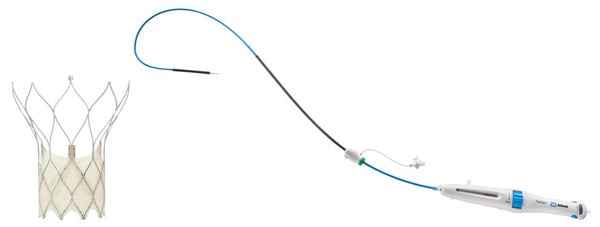
Figure 1. Portico with FlexNav TAVI system.
Additionally, the hydrophilic coating on the inserted length of the delivery system significantly reduces the surface frictional properties of the catheter and augments easy tracking through tortuous vessels. Lastly, ease-of-use improvements are accomplished through a redesigned handle with automation of the deployment lock mechanism, increased mechanical advantage (less force per turn), and the addition of a dedicated macro slide feature, all serving to simplify the user interface and provide a streamlined workflow. In total, the FlexNav delivery system represents a significant advancement and greatly simplifies and improves implantation of the Portico valve.
Improved placement accuracy and stability
Improvements in placement accuracy and stability were achieved with the addition of a stability layer to the main catheter assembly (Figure 2). This additional layer encapsulates the primary outer shaft, which contains the valve capsule and thus eliminates all motion of the delivery system at the vessel access site during deployment.
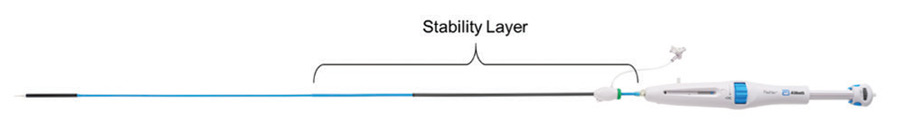
Figure 2. Stability layer.
By eliminating this motion, the user does not need to manipulate the delivery system at the access site to obtain valve placement accuracy because there is no tendency for the valve to “dive” into the left ventricle during implantation.
Low insertion profile
To maintain a low insertion profile, an integrated introducer sheath is incorporated into the delivery system proximal to the valve capsule (Figure 3).
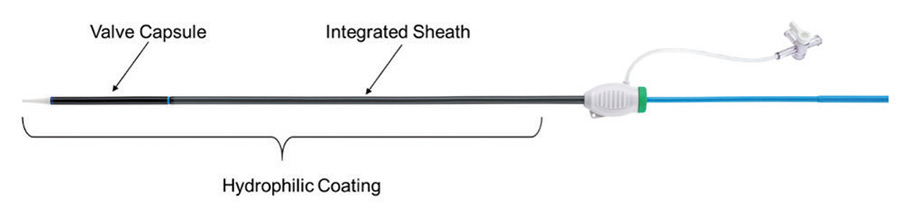
Figure 3. Integrated sheath and hydrophilic coating.
This integrated sheath has the same outer diameter as the delivery catheter, offering 14-F (6 mm) and 15-F (6.3 mm) equivalent sheath diameters for the two delivery system sizes (small size for 23- and 25-mm valves and large size for 27- and 29-mm valves) and allowing for insertion of the delivery system without the use of a separate introducer sheath. Additionally, the hydrophilic coating enhances tracking of the delivery system into the access vessel.
Ease of use
The last main area of focus for the FlexNav delivery system was an overall improvement in ease of use, which was largely achieved through a redesign of the control handle (Figure 4).
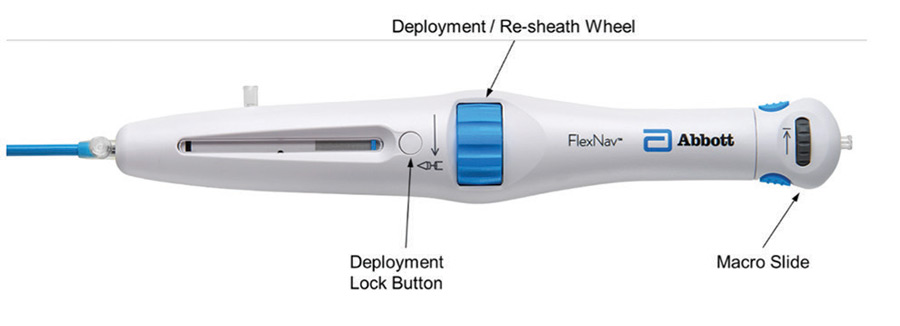
Figure 4. FlexNav control handle.
The primary deployment wheel mechanism was maintained; however, mechanical design improvements were made to give the deployment wheel an increased mechanical advantage and smoother action, providing a more consistent tactile feel during deployment and, if necessary, the ability to recapture the valve. In addition to the improved deployment mechanisms, the deployment lock button was redesigned to automatically engage during use, eliminating the need to manually engage the lock during device preparation and resheathing, thus reducing the overall number of steps to use the device. Lastly, a pullback handle (macro slide) was incorporated to clearly delineate the device closure mechanism from the deployment/recapture wheel and allow for atraumatic recapture of the distal tip of the catheter into the valve capsule after final valve release.
Apart from the redesigned control handle, additional ease-of-use improvements arise from the previously mentioned stability layer, which eliminates the need for manipulation to maintain implant position and reliable release of the valve from the delivery system on full deployment.
Deliverability
Excellent deliverability of the FlexNav system is achieved by leveraging the highly flexible shaft design of the original Portico delivery system as well as the Portico valve, which has less metal by design. This delivery system shaft was optimized for reliable resheathability without the need for significant metallic support structures, resulting in a very trackable system. For FlexNav, the addition of the integrated sheath and hydrophilic coating is intended to optimize deliverability even further. These features together are designed to easily traverse tortuous anatomy leading up to the aortic arch as well as horizontal aortas.
Summary
The Portico transcatheter valve system has been used to treat nearly 15,000 patients with aortic stenosis and continues to gain momentum in an increasingly crowded market.
Information contained herein for DISTRIBUTION outside of the U.S. ONLY.
Check the regulatory status of the device in areas where CE marking is not the regulation in force.
Page published on May 2020



