29 Mar 2021
Long term data on Tavi Durability
Sponsored by Medtronic
Medtronic TAVI platform engineered for today and tomorrow. With its supra-annular, self-expanding valve frame, the Medtronic TAVI platform has consistently sustained large EOAs and low mean gradients over time – all of which lead to low rates of Structural Valve Deterioration (SVD).1-4
How is SVD defined?
- As the treatment modalities of aortic stenosis have changed, so have the definitions of failure
- Whereas with surgical valves the structural failure was confirmed on explant during the redo surgery, TAVI relies on echographic interrogation of the valve to determine whether the hemodynamics remain stable, signaling the valve’s durability
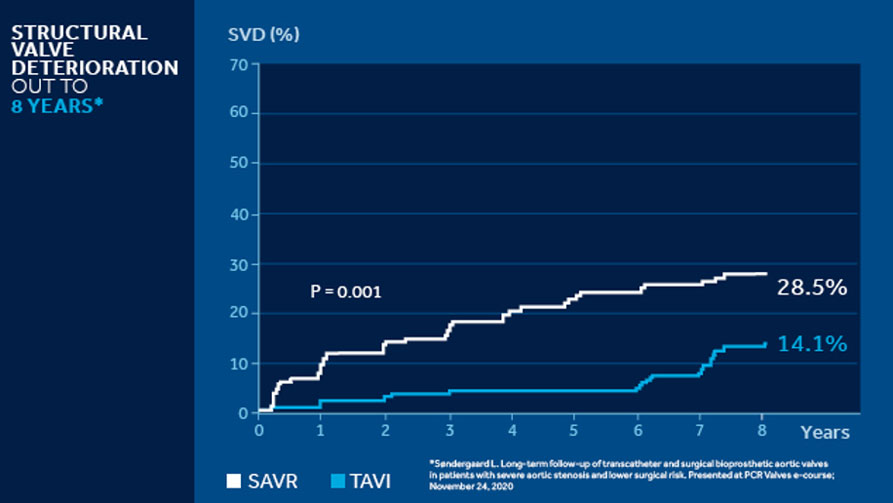
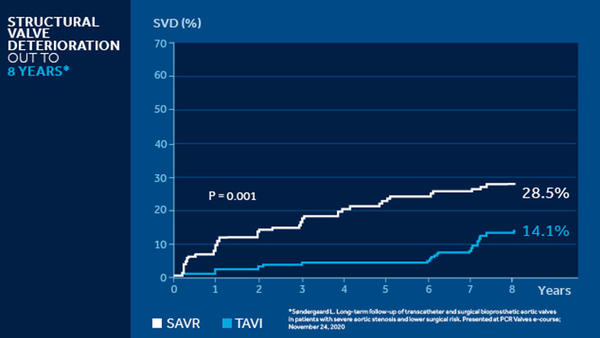
NOTION 8 years3
The NOTION trial is a multicenter, randomized, head-to-head comparison of CoreValve TAVI versus SAVR followed out to 8 years in lower surgical risk patients ≥70 years of age that are eligible for surgery. TAVI had significantly less hemodynamic SVD out to 8 years.
The NOTION 8 years data demonstrates excellent SVD rates in a lower surgical risk patient population. Perhaps most importantly, the data provides a signal of durability for the CoreValve platform versus SAVR.
To learn more about how should the clinical data on TAVI durability impact the clinical practice, watch new webinar
Moderator
Speakers
Objectives:
Watch this PCR Webinar if you want to:
- Know the available literature on surgical and transcatheter aortic valve durability
- Get an update of the clinical impact of sub clinical valve thrombosis
- Learn how haemodynamics after TAVI impact valve durability
Stay tuned for the next TAVI webinar session about “How to reduce PPM rate with cusp overlap technique?” on 26th of April. Register here
Have you missed previous webinars? No problem! Here you can find all the previous videos.
- Abdel-Wahab M, et al. Five-year outcomes after TAVI with balloon-expandable vs. self-expanding valves: Results from the CHOICE randomised clinical trial. Presented at EuroPCR 2019; Paris, France.
- Deutsch MA, Erlebach M, Burri M, et al. Beyond the five-year horizon: long-term outcome of high-risk and inoperable patients undergoing TAVR with first-generation devices. EuroIntervention. May 20, 2018;14(1):41-49.
- Søndergaard L. Long-term follow-up of transcatheter and surgical bioprosthetic aortic valves in patients with severe aortic stenosis and lower surgical risk. Presented at PCR Valves e-Course; November 24, 2020.
- Testa L, et al. Long-term clinical outcome and performance of transcatheter aortic valve replacement with a self-expandable bioprosthesis. European Heart Journal. May 21, 20;41(20):1876-1886.
See the CoreValve™ Evolut™ R and the CoreValve™ Evolut™ PRO device manuals for detailed information regarding the instructions for use, indications, contraindications, warnings, precautions, and potential adverse events. For further information, contact your local Medtronic representative and/or consult the Medtronic website at medtronic.eu.
This PCR Webinar on TAVI is available thanks to the support of Medtronic.
PCR develops independent educational programmes.

Page originally published on March 2021