26 Oct 2017
Boston Scientific: Lotus™ Valve System
Transcatheter aortic valve devices
The LOTUS™ Valve System is a controlled mechanical expansion TAVI technology, designed to provide unmatched freedom from PVL, precise delivery and deployment.
The LOTUS™ Valve System is a registered trademark of Boston Scientific. Boston Scientific Corporation has finished the acquisition of Symetis SA. Boston Scientific now sells the ACURATE TA™ and ACURATE neo™/TF™ valve systems.
Lotus™ Valve System Introduction
The Lotus Valve Transcatheter Aortic Valve Replacement (TAVR) device offers precise positioning and placement, offering physicians unprecedented control.
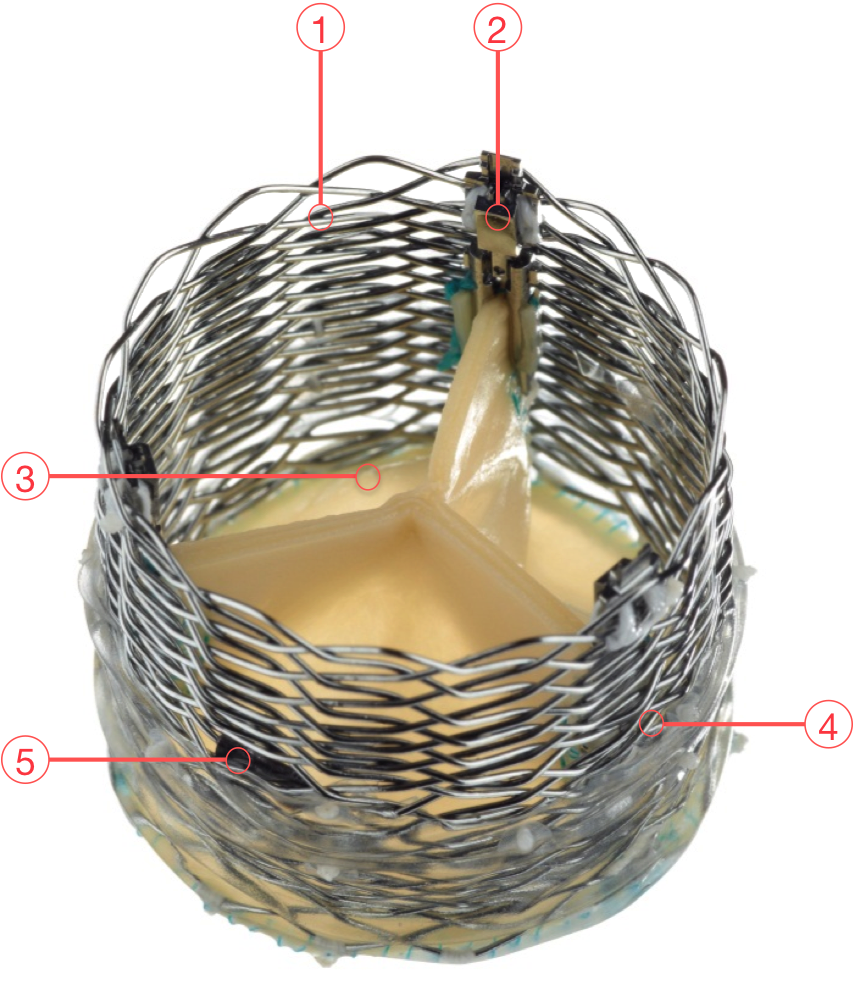
Figure 55. The Lotus Valve System
1 - Braided Nitinol frame
2 - Locking mechanism
3 - Bovine pericardium
4 - Adaptive seal TM
5 - Central radiopaque positioning marker
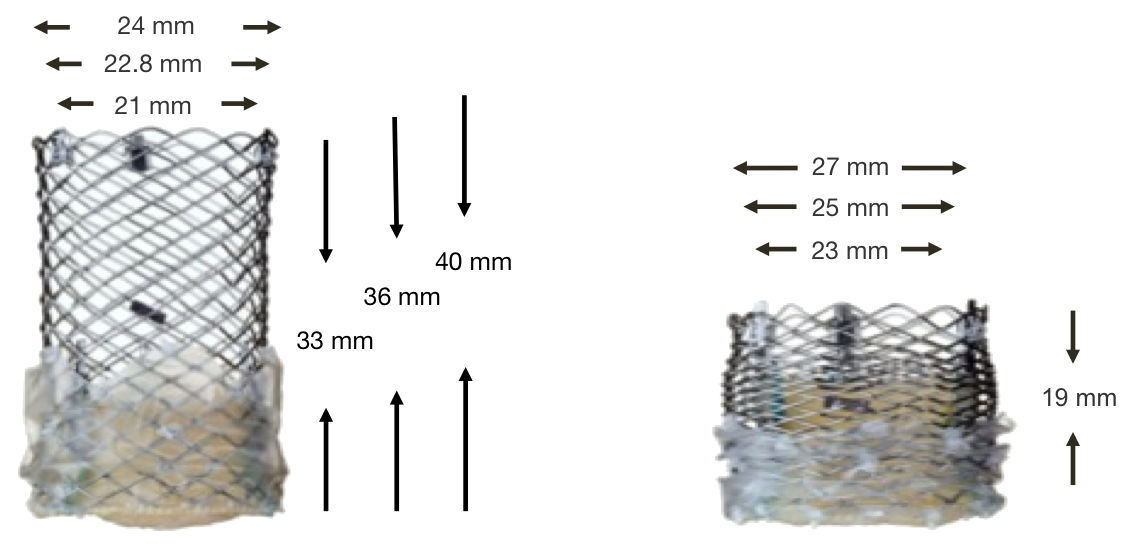
The Lotus Valve System dimensions
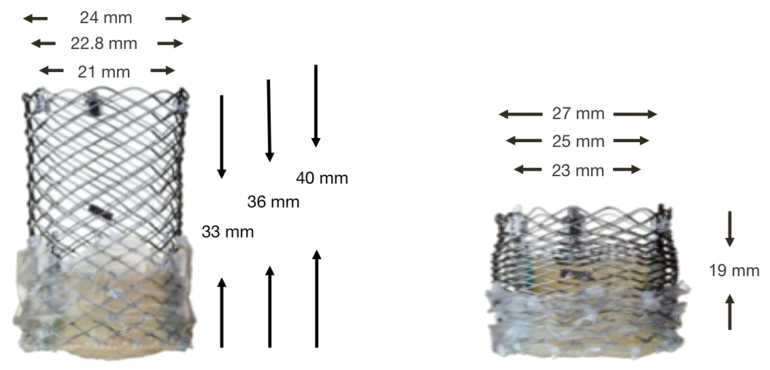
Figure 56. Lotus Valve System (dimensions)
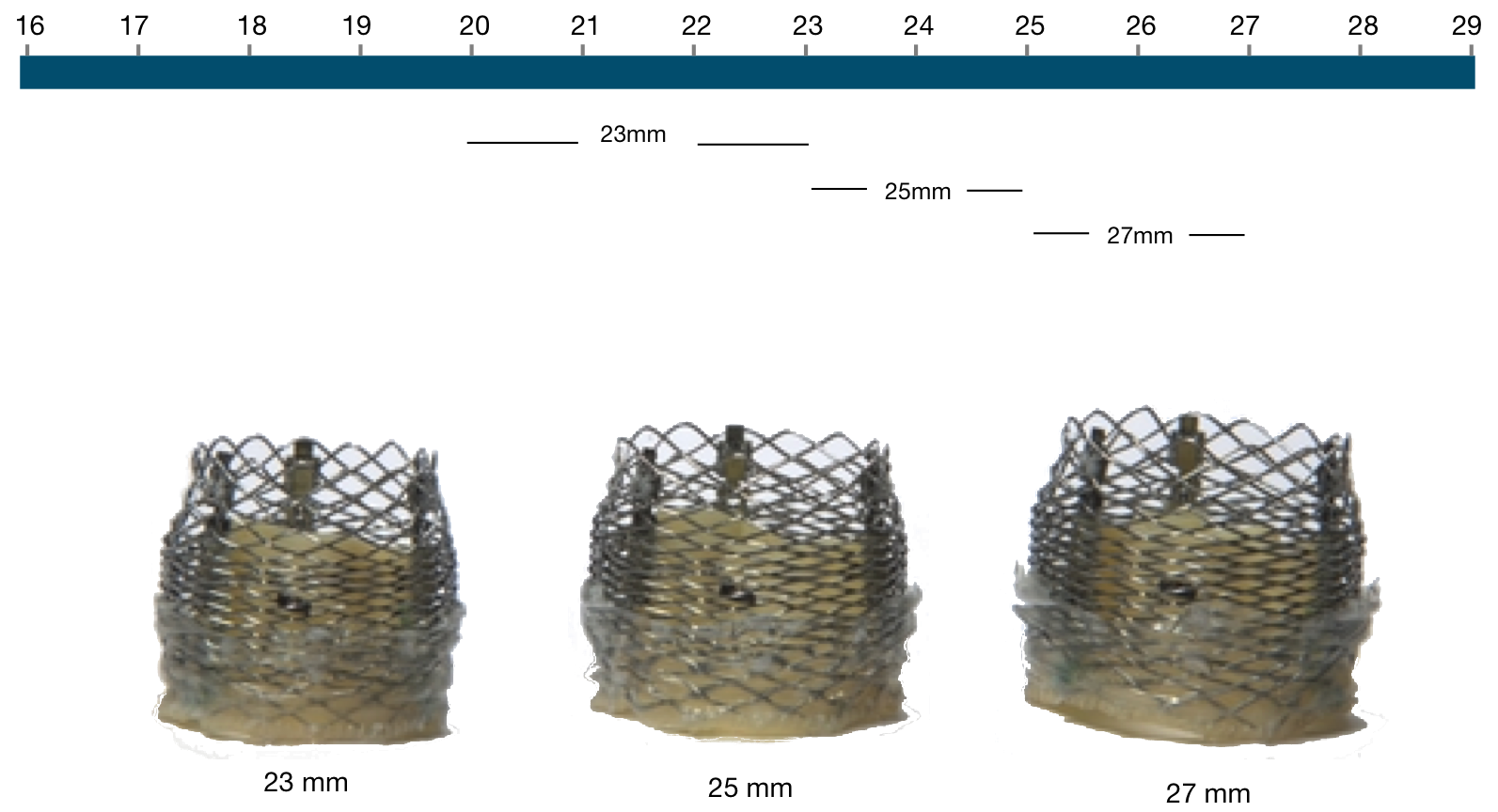
The Lotus Valve System native annulus diameter
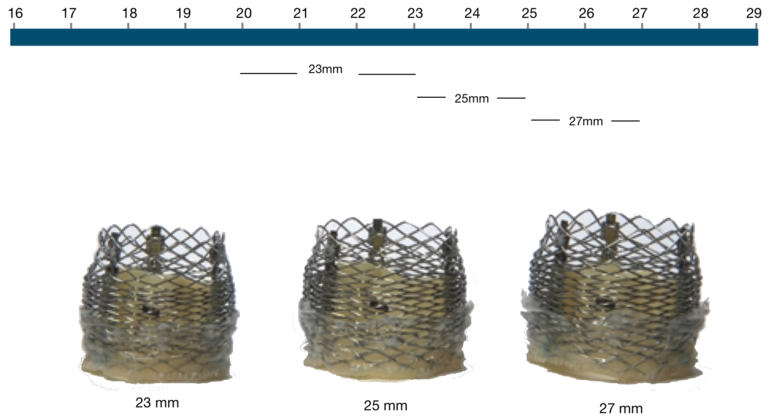
Figure 57. The Lotus Valve System (native annulus diameter)
Lotus Valve | Native annulus diameter |
|---|---|
23 mm | 20-23 mm |
25 mm | 23-25 mm |
27 mm | 25-27 mm |
Boston Scientific Lotus™ Valve System animation
Lotus™ Valve System
General characteristics:
Design | Controlled mechanical expansion |
Frame | Braided nitinol |
Leaflets | Bovine pericardium |
Valve size (mm) | 23 mm, 25mm, 27 mm |
Delivery system diameter - 1 (Figure 58), 2 (Figure 59), 3 (Movie 27) | 18 Fr, 20 Fr |
Implantation access | Transfemoral (Movie 14) |
Repositionable | Yes |
Retrievable after being fully deployed | Yes |
Additional cuff to reduce AR | Yes |
Anchoring mechanism | No |
Tactile feedback during deployment | No |
Large stent cell design | No |
Markers to facilitate deployment | Yes |
Motorised delivery system | No |
Anticalcification technology | Yes |
CE mark status: Yes - 28/10/2013
Special features:
- The Lotus™ Valve System has been designed to give physicians total control during TAVI procedures through the ability to fully reposition, retrieve and redeploy, even after full deployment and prior to release
- Controlled release of the valve
- Adaptive Seal™ promotes annular sealing and prevents paravalvular leak
Lotus angiogram implantation:
- Boston Scientific Lotus Valve System: Angiograms
- Boston Scientific Lotus Valve System: Phantom model implantation
- Boston Scientific Lotus Valve System: 3D rotational animation
For more information about the LOTUS Edge Valve System, please click here