6 results for «1925»
6 results
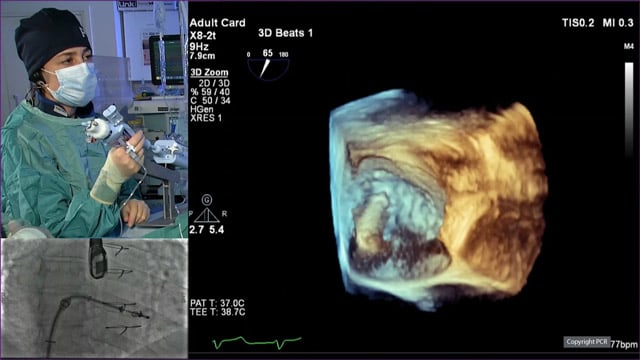
Transcatheter tricuspid valve replacement - LIVE case
Recommended by PCR
15 May 2024 – From EuroPCR 2024
An 82-year-old male with a history of STEMI in 2013, stroke in 2018, cancer, and permanent AF, presented with acute heart failure with predominant right-side failure. Severe tricuspid regurgitation was identified.
The operators implanted an Evoque valve percutaneously under TEE guidance and general anaesthesia via the venous...
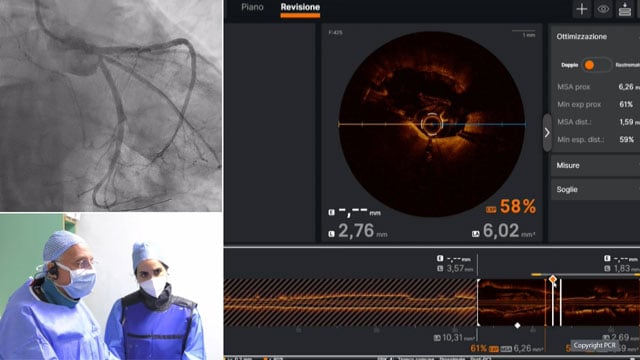
Imaging-guided left main PCI - LIVE case
Recommended by PCR
17 May 2024 – From EuroPCR 2024
A 53-year-old male with a history of advanced malignancy (lung adenoma) and mid LAD PCI last year, along with multiple risk factors (hypertension, dyslipidemia, diabetes, and smoking), presented with angina and a positive stress test and CT. The angiography and CT showed significant left main stenosis...
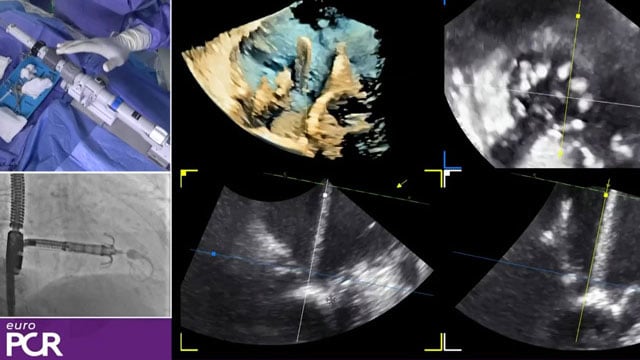
Advanced mitral TEER - LIVE case
Recommended by PCR
24 Nov 2024 – From PCR London Valves 2024
Join us for a LIVE case followed by a recorded case highlighting advanced treatments for severe secondary mitral regurgitation:
- A 75-year-old man with permanent AF, a history of CABG, and symptomatic severe secondary MR (atrial and ischemic causes) with low LV function (45%) underwent mitral repair with...
Left main - LIVE Case
Recommended by PCR
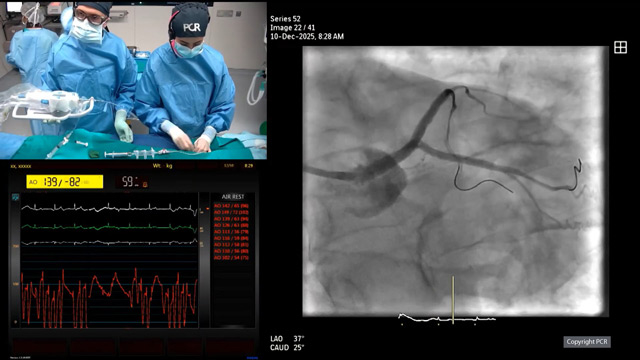
10 Dec 2025 – From GulfPCR-GIM 2025
This session delivers an in-depth exploration of left main coronary artery intervention, featuring a live educational case from the Mohammed Bin Khalifa Bin Salman Al Khalifa Specialist Cardiac Centre in Awali, Bahrain. Attendees gain insights into the pivotal role of computed tomography and intravascular imaging in...
Transcatheter management of failed THV - LIVE case
Recommended by PCR
09 Feb 2025 – From PCR Tokyo Valves 2025
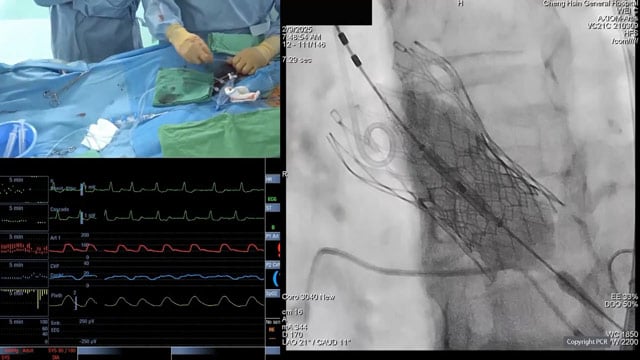
An 84-year-old woman with diabetes mellitus, frailty (5/6), preserved LV function, and aortic stenosis previously treated with CoreValve 26 mm in 2016, presented with symptomatic degenerative stenosis of the valve.
A Sapien 3 (23 mm) was implanted without predilatation but with post-dilatation under general anaesthesia, using cerebral...
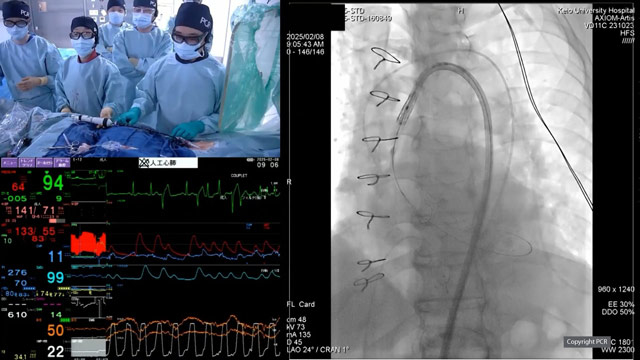
Transcatheter options for degenerated surgical aortic valves - LIVE case
Recommended by PCR
08 Feb 2025 – From PCR Tokyo Valves 2025
A 77-year-old male with a history of SAVR in 2011 (Magna Ease 23) and moderate chronic kidney disease, with preservedLV function, presented with symptomatic severe aortic regurgitation and stenosis, associated with severe MR.
A Sapien 3 Ultra Resilia 23 valve was implanted under local anesthesia, with hemodynamic...