Predicting the unpredictable: application of predictive modeling in complex transcatheter aortic valve replacement
Supported by the EuroIntervention Journal
Authors*
Alfredo Redondo Diéguez MD1, Taylor Sirset-Becker, MS2, Belén Cid Álvarez PhD1, Xabier Irazusta Olloquiegui MD1, Jose R. González-Juanatey PhD1, Ramiro Trillo Nouche MD1, and Lakshmi Prasad Dasi, PhD3
Case summary
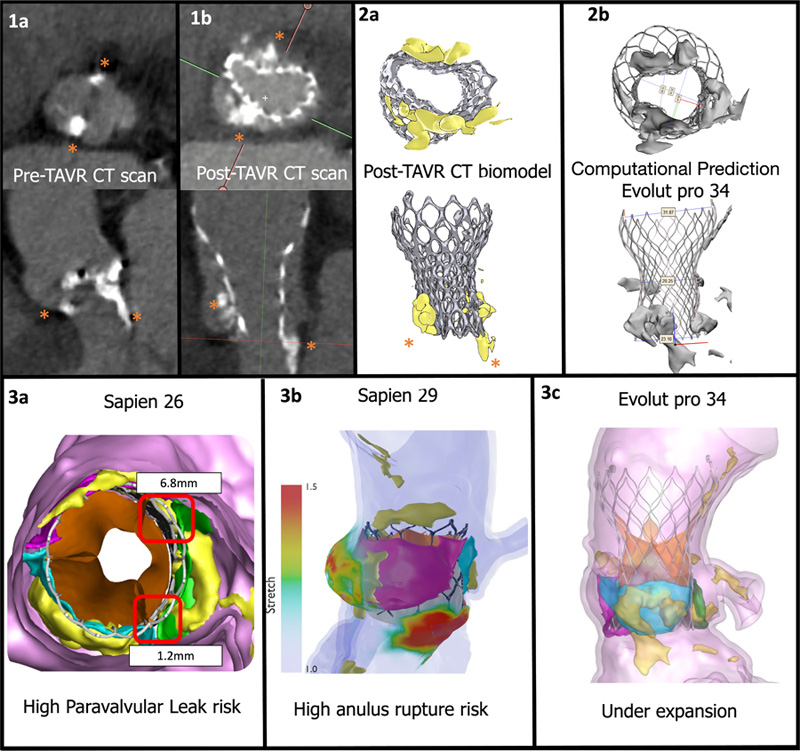
A 76-year-old male patient at low surgical risk underwent transcatheter aortic valve replacement (TAVR). The computed tomography (CT) prior to the procedure showed a heavily calcified aortic valve with prominent calcific nodules in the non-coronary cusp, the right coronary cusp, and in the left ventricular outflow tract (LVOT) under the non-coronary and left coronary sinuses (Figure 1a, Video 1).
A Safari extra-small wire (Boston scientific) was placed in the left ventricle. Predilatation with a True Dilatation 24 mm balloon (Bard Vascular Inc) was performed. In a left-to-right cusp overlap (2-cusp) projection, a Medtronic Evolut Pro 34 (Medtronic) was implanted (Video 1).
The operator was unable to retrieve the delivery catheter as the nose cone was trapped in the valve inflow. A mild post-dilatation was performed with a 20 mm Cristal Balloon (Ballt), 8-French compatible, used through the contralateral femoral access to facilitate release of the nose cone.
Finally, the delivery catheter was successfully retrieved. Angiography did not reveal any under-expansion of the device in different projections. Control aortography revealed no paravalvular leaks. Despite this, the operator decided not to proceed with post-dilatation, due to anulus rupture risk and high implant height.
In the routine echocardiographic exam prior to discharge, high gradients were detected (Video 2). To further understand valve expansion, a CT scan was performed, which revealed a significant antero-posterior under-expansion at the prosthesic valve inflow, due to very prominent calcific nodules in the non-coronary and right coronary sinuses, and in the LVOT (Figures 1b and 2a). The heart team dismissed valvular post-dilatation due to the calcification at the LVOT and the potential risk of annular rupture.
In our experience, despite the described calcification, valve under-expansion was not predictable using traditional 2D CT analysis. Retrospectively, a computational predictive analysis of the TAVR implant was conducted by DASI Simulations, LLC (Dublin, OH, USA), using the pre-TAVR CT scan. This computational analysis consists in a reduced-order analysis method rooted in physics and artificial intelligence, that has the ability to simulate the precise deformation of the prosthesis and the patient’s tissue. This enabled the Heart Team to review the case with a 3D virtual implant1,2.
The purpose of conducting this retrospective analysis was to assess whether the complication would have been predictable if the simulations had been conducted during the pre-procedural planning process. Secondly, we wanted to understand whether a different TAVR prosthesis would have resulted in a different clinical outcome for this patient.
Implant simulation with a virtual Evolut Pro 34 resulted in similar frame deformation with maximal and minimal diameters similar to those of the post-TAVR, CT scan with opposing calcium nodules at the sides of the minimal diameter of the prosthesis (Figure 2b and 3b, and Video 2).
Additionally, 26 and 29 SAPIEN 3 valves (Edwards Lifesciences) implantations were simulated. The simulation results demonstrated that the former, with an under-expansion of 2.44%, would have resulted in paravalvular leak, with gaps up to 6 mm (Figure 3a); the latter would have applied a high stress at the anulus, which could have led to its rupture, with a maximal stretch tissue of 1.6 mm (Figure 3b). This is due to the high surface stretch caused by the prosthesis interacting with the calcific nodules in the patient’s aortic root, with minimal space to accommodate the prosthesis.
We believe that this case highlights the potential add-on value of reduced-order computational modeling over standard CT scan analysis due to its ability to predict the interaction of different devices or sizes with the patient-specific anatomy. This is of particular interest for intermediate- to low-risk TAVR candidates, for whom conventional surgery might be a preferred option if, based on anatomy, TAVR complications cannot be ruled out. It is also beneficial when planning aortic valve intervention for patients with bicuspid aortic valves, as the dynamic interaction between the valve and the aortic root can be more complex, due to the higher levels of calcium seen in these patients.

Figure 1a: Pre-TAVR CT scan highlighting the location of the calcium nodules (asterisks) at the anulus and LVOT.
Figure 1b: Post-TAVR CT scan.
Comparison of frame deformation between CT scan derived biomodel (Figure 2a) and the computational prediction implant of a Evolut pro+ 34 (Figure 2b).
Potential complications of different prostheses detected by computational prediction (aortic native leaflets are represented in blue, green and violet):
Sapien 26 prosthesis simulation (Figure 3a), red squares locate gaps where paravalvular leak is expected.
Sapien 29 simulation with an overlayed stretch colour map (Figure 3b), colours at the aortic root represent the stretch of the tissue in millimetres, red zones are at risk of anulus rupture.
Evolut 34 simulation (Figures 2b and 3c) shows under-expansion of the device.
Supplementary materials
References
- Esmailie F, Razavi A, Yeats B, Sivakumar SK, Chen H, Samaee M, Shah IMA, Veneziani A, Yadav P, ThouraniVH and Dasi LP. Biomechanics of Transcatheter Aortic Valve Replacement Complications andComputational Predictive Modeling. Structural Heart-the Journal of the Heart Team. 2022;6.
- Sirset, T.N., et al., CRT-700.11 Peak Areal Stretch Location as a Variable to Predict Aortic Root Rupture Risk Using Pre-Procedural Computational Modeling. JACC: Cardiovascular Interventions, 2023. 16(4_Supplement): p. S86-S86.
Affiliations*
- Cardiology, University Hospital of Santiago de Compostela. IDIS. CIBERCV
- The Ohio State University College of Medicine, Columbus, Ohio
- Georgia Institute of Technology and Emory University, Atlanta, Georgia
Conflicts of interest
- Ramiro Trillo is proctor for Boston scientific and Medtronic.
- Lakshmi Dasi and Taylor Sirset-Becker have relationship with Dasi Simulations and have patents on predictive computational modeling.






No comments yet!