12 May 2020
Implantation of the Portico With FlexNav TAVI System in a patient with aortic stenosis, severe renal impairment, and diffuse iliac atherosclerosis
Sponsored by Abbott
Real-world case report illustrating the benefits of the Portico with FlexNav TAVI system.
Portico with FlexNav TAVI system: enhancing innovative technology to optimize patient outcomes and physician experience
A case report by Sinny Delacroix, MD, PhD; Ramesh G. Chokka, MD and Stephen G. Worthley, MBBS, PhD, FRACP
Patient presentation
This case describes a 90-year-old woman with comorbidities that included type 2 diabetes, morbid obesity, hypertension, mixed mitral valve disease, chronic kidney disease, and hyperlipidemia, as well as a history of transient ischemic attack, pacemaker implantation for trifascicular block, breast cancer with right lumpectomy, and bilateral total knee replacement. She presented with worsening shortness of breath over several months and was assessed for transcatheter aortic valve implantation (TAVI) considering the high surgical risk due to her medical history. She lived alone, was independent with her activities of daily living, and was mobile with the use of a four-wheeled walker and an electric wheelchair. She had a Mini-Mental State Examination score of 28/30 and a Society of Thoracic Surgeons score of 7.9%.
Transthoracic echocardiography (TTE) revealed severe aortic stenosis with a left ventricular ejection fraction
(LVEF) of 55%. Mild mitral and tricuspid regurgitation was also noted. Coronary angiography revealed mild-to-moderate disease in the dominant left coronary artery and no disease in the nondominant right coronary artery. A coronary height of 8.7 mm in the diseased left coronary artery added to the complexity of the case. The aortic valve area was 0.8 cm2, with a mean gradient of 36 mm Hg, Vmax of 4 m/s, and a dimensionless performance index of 0.2, which is indicative of severe aortic stenosis.
CT assessments using 3mensio software (Pie Medical Imaging) showed an annular area of 543 mm2 and a perimeter of 83.5 mm. The left and right coronary artery heights were 8.7 mm and 20.4 mm, respectively. Vascular access was challenging due to the relatively small femoral diameters of 5.4 mm and 6.1 mm. Diffuse minor atherosclerosis was also noted in the iliac arteries, which were quite tortuous as well (Figure 1).
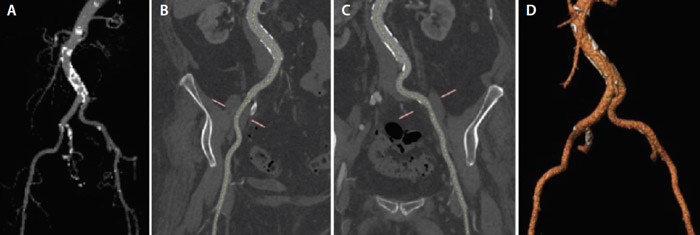
Figure 1. Contrast CT image of the iliofemoral arteries (A), snake view of the right iliac (B), snake view of left Iliac (C), and three-dimensionally rendered image of the iliofemoral arteries (D).
Procedure description and results
A 29-mm Portico valve (Abbott) was deployed under local anesthesia and conscious sedation using the FlexNav delivery system (Abbott) via a transfemoral access route (Figure 2A and 2B).
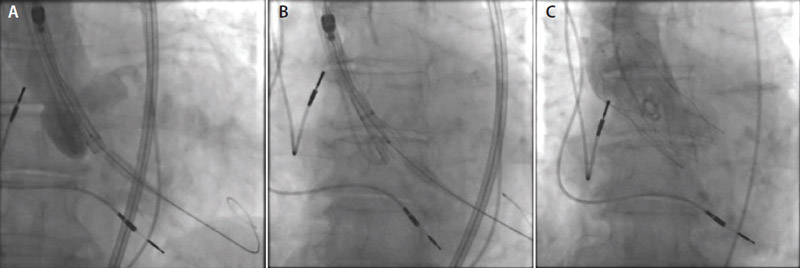
Figure 2. FlexNav delivery system across the aortic valve (A). Initial stages of Portico valve deployment (B). The Portico valve in position (C).
Immediately after the procedure, the patient had no residual aortic valve gradient or paravalvular aortic regurgitation on cine aortography and TTE. Access was achieved without complication using 6- and 14-F sheaths in the left and right femoral arteries, respectively, and the access was preclosed with two Perclose ProGlide devices (Abbott). The aortic valve was crossed with a 6-F Amplatzer left 1 guide catheter followed by a 6-F pigtail catheter. Valvuloplasty with a 23-mm Cristal balloon (Balt) was performed under rapid pacing via the existing pacemaker. The 14-F sheath was then removed and the FlexNav delivery system with its 15-F integrated sheath was introduced. This was performed quite easily, despite the tortuous iliac artery anatomy. A 29-mm Portico valve was then deployed without pacing. The frictionless sheath permitted stable delivery of the valve without movement into the ventricle during valve release, and thus we were able to achieve a stable high position with an excellent result (Figure 2C). The final position of the ventricular aspect of the valve frame was 0 mm below the noncoronary cusp and 2 mm below the left coronary cusp. Hemostasis was obtained with the Perclose ProGlide devices.
The postoperative course was uneventful with predischarge TTE showing a well-seated Portico valve and no aortic regurgitation. The patient was discharged 1 day after the procedure on a regimen of aspirin and clopidogrel.
At the 30-day postoperative follow-up, the patient was significantly less short of breath than she was prior to the procedure but remained in New York Heart Association class II. Her TTE findings, from an aortic valve perspective, remained unchanged from discharge with a well-seated valve, no paravalvular leaks, and normal hemodynamics. There were no significant issues after discharge.
At the 6-month follow-up, the patient expressed that although her exertional dyspnea had significantly improved, she continued to experience some shortness of breath with exertion that could be due to her obesity and lack of physical fitness. TTE demonstrated a well-seated Portico valve with trivial paravalvular leak and a normal LVEF of 55%. She had no other complaints. Clopidogrel was ceased, but she continued to take aspirin.
Discussion
Over the last decade, evidence from several clinical trials has propelled the evolution of TAVI in the treat-ment of severe aortic stenosis.1-7 It is currently the preferred treatment modality for patients with severe symptomatic aortic stenosis, and studies have shown that the procedure is noninferior or even superior to standard therapies in intermediate-risk patients.8,9 More recently, based on the results of the PARTNER 3 trial, the FDA has approved an expanded indication for several transcatheter heart valves to include patients with severe aortic stenosis, even at low surgical risk.5
The Portico valve consisting of a self-expanding nitinol frame started its clinical use in 2012 and has gained regulatory approval in Europe for sheathless introduction of the valve, providing physicians with the ability to use the valve in patients with complex anatomies. The large stent cell sizes of the valve with minimal flaring and the valve's intra-annular design provide the added advantages of improved valve positioning and hemodynamic stability. The new FlexNav delivery system provides the added advantage of stable, controlled deployment of the valve, which permits very accurate placement of the valve frame. By permitting safe, accurate deployment, the valve can be more reliably deployed at a target depth of 3 mm, which potentially reduces risk of pacemaker implantation and may also reduce paravalvular leak. The smaller French size (14-F equivalent for the 23/25-mm valves and 15-F equivalent for the 27/29-mm valves) may reduce the vascular complication rate and could potentially treat patients with some compromise of the iliac and femoral arteries that would not be accessible with traditional TAVI delivery systems.
To watch an accompanying video, please view this article at https://citoday.com/.
References
- Gleason TG, Reardon MJ, Popma JJ, et al. 5-year outcomes of self-expanding transcatheter versus surgical aortic valve replacement in high-risk patients. J Am Coll Cardiol. 2018;72:2687-2696.
- Kapadia SR, Leon MB, Makkar RR, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485-2491.
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607.
- Leon MB, Smith CR, Mack MJ, et al Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609-1620.
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695-1705.
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198.
- Webb JG, Doshi D, Mack MJ, et al. A randomized evaluation of the SAPIEN XT transcatheter heart valve system in patients with aortic stenosis who are not candidates for surgery. JACC Cardiovasc Interv. 2015;8:1797-1806.
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739-2791.
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252-289.
Sinny Delacroix, MD, PhD
Royal Adelaide Hospital
Adelaide, South Australia, Australia GenesisCare
Alexandria, New South Wales, Australia Disclosures: None.
Ramesh G. Chokka, MD
Royal Adelaide Hospital
Adelaide, South Australia, Australia GenesisCare
Alexandria, New South Wales, Australia Disclosures: None.
Stephen G. Worthley, MBBS, PhD, FRACP
Royal Adelaide Hospital
Adelaide, South Australia, Australia GenesisCare
Alexandria, New South Wales, Australia
Disclosures: Research grants from Abbott.
Portico With FlexNav TAVI System: Enhancing Innovative Technology to Optimize Patient Outcomes and Physician Experience
By Mike Morrissey
The original Portico transcatheter aortic valve implantation (TAVI) system (Abbott) comprises a transcatheter valve and a delivery system used to navigate and deploy the valve into position at the native aortic annulus. Throughout the initial clinical experience with the Portico system, several aspects were identified as major strengths. These included the low profile and overall flexibility of the delivery system; ease of tracking the system over the aortic arch; intra-annular valve positioning for hemodynamic stability during deployment; recapturability, repositionability, and retrievability of the valve; and large-cell geometry for easy coronary access postimplantation.
Over time, additional areas for improvement were identified as physicians expressed a need for sheathless vascular access, valve placement accuracy, and overall improvements in ease of use.
Latest-generation TAVI system
As a response to these customer requests, the FlexNav delivery system (Abbott; Figure 1) was developed to address improvement needs while maintaining the positive performance aspects of the original Portico system (see the Technology Design Goals Met By the FlexNav Delivery System sidebar). The FlexNav delivery system incorporates a stability layer to the catheter, reducing the amount of manipulation required at the access site and providing predictable, accurate, and stable implantation of the valve at the annulus. The addition of an integrated sheath onto the catheter allows easy, sheathless access into the vasculature and maintains a low insertion profile, allowing access into vessels as small as 5 mm for the small system and 5.5 mm for the large system.
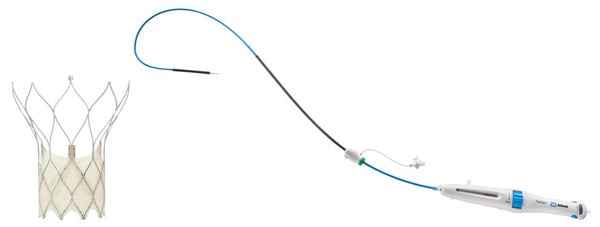
Figure 1. Portico with FlexNav TAVI system.
Additionally, the hydrophilic coating on the inserted length of the delivery system significantly reduces the surface frictional properties of the catheter and augments easy tracking through tortuous vessels. Lastly, ease-of-use improvements are accomplished through a redesigned handle with automation of the deployment lock mechanism, increased mechanical advantage (less force per turn), and the addition of a dedicated macro slide feature, all serving to simplify the user interface and provide a streamlined workflow. In total, the FlexNav delivery system represents a significant advancement and greatly simplifies and improves implantation of the Portico valve.
Improved placement accuracy and stability
Improvements in placement accuracy and stability were achieved with the addition of a stability layer to the main catheter assembly (Figure 2). This additional layer encapsulates the primary outer shaft, which contains the valve capsule and thus eliminates all motion of the delivery system at the vessel access site during deployment.
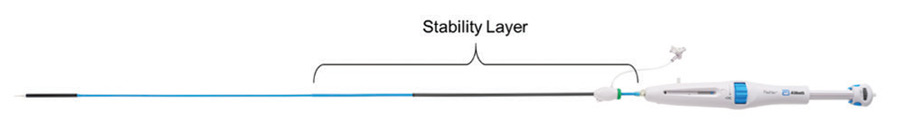
Figure 2. Stability layer.
By eliminating this motion, the user does not need to manipulate the delivery system at the access site to obtain valve placement accuracy because there is no tendency for the valve to “dive” into the left ventricle during implantation.
Low insertion profile
To maintain a low insertion profile, an integrated introducer sheath is incorporated into the delivery system proximal to the valve capsule (Figure 3).
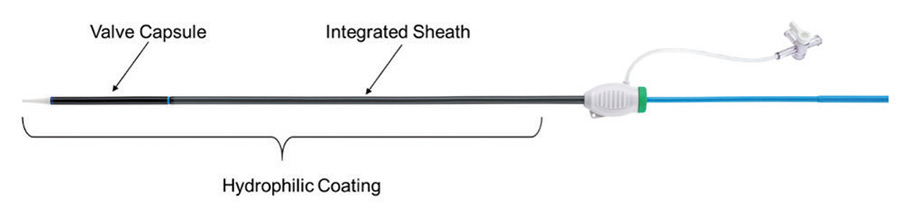
Figure 3. Integrated sheath and hydrophilic coating.
This integrated sheath has the same outer diameter as the delivery catheter, offering 14-F (6 mm) and 15-F (6.3 mm) equivalent sheath diameters for the two delivery system sizes (small size for 23- and 25-mm valves and large size for 27- and 29-mm valves) and allowing for insertion of the delivery system without the use of a separate introducer sheath. Additionally, the hydrophilic coating enhances tracking of the delivery system into the access vessel.
Ease of use
The last main area of focus for the FlexNav delivery system was an overall improvement in ease of use, which was largely achieved through a redesign of the control handle (Figure 4).
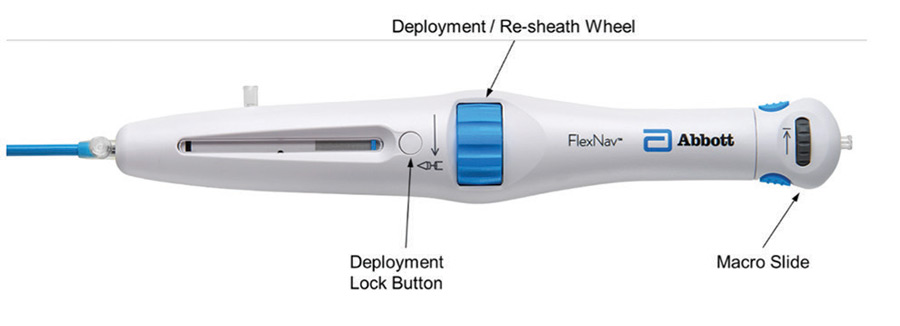
Figure 4. FlexNav control handle.
The primary deployment wheel mechanism was maintained; however, mechanical design improvements were made to give the deployment wheel an increased mechanical advantage and smoother action, providing a more consistent tactile feel during deployment and, if necessary, the ability to recapture the valve. In addition to the improved deployment mechanisms, the deployment lock button was redesigned to automatically engage during use, eliminating the need to manually engage the lock during device preparation and resheathing, thus reducing the overall number of steps to use the device. Lastly, a pullback handle (macro slide) was incorporated to clearly delineate the device closure mechanism from the deployment/recapture wheel and allow for atraumatic recapture of the distal tip of the catheter into the valve capsule after final valve release.
Apart from the redesigned control handle, additional ease-of-use improvements arise from the previously mentioned stability layer, which eliminates the need for manipulation to maintain implant position and reliable release of the valve from the delivery system on full deployment.
Deliverability
Excellent deliverability of the FlexNav system is achieved by leveraging the highly flexible shaft design of the original Portico delivery system as well as the Portico valve, which has less metal by design. This delivery system shaft was optimized for reliable resheathability without the need for significant metallic support structures, resulting in a very trackable system. For FlexNav, the addition of the integrated sheath and hydrophilic coating is intended to optimize deliverability even further. These features together are designed to easily traverse tortuous anatomy leading up to the aortic arch as well as horizontal aortas.
Summary
The Portico transcatheter valve system has been used to treat nearly 15,000 patients with aortic stenosis and continues to gain momentum in an increasingly crowded market.
Information contained herein for DISTRIBUTION outside of the U.S. ONLY.
Check the regulatory status of the device in areas where CE marking is not the regulation in force.
Page published on May 2020



