12 May 2020
TAVI in a patient with tortuous iliac anatomy and calcification
Sponsored by Abbott
Real-world case report illustrating the benefits of the Portico with FlexNav TAVI system.
Portico with FlexNav TAVI system: enhancing innovative technology to optimize patient outcomes and physician experience
A case report by Antony Walton, MBBS, FRACP, FCSANZ
Patient presentation
An 85-year-old man presented with progressive symptomatic aortic stenosis (New York Heart Association class II) and reported progressive dyspnea and angina. His clinical history included coronary artery bypass grafting
(left internal mammary artery [LIMA] to left anterior descending [LAD] artery, saphenous vein graft [SVG] to LAD-D1, and SVG to posterior descending artery [PDA]), peripheral vascular disease treated with an endovascular stent graft (left femoral artery aneurysm repair), as well as hypertension and atrial fibrillation. There was moderate renal impairment with an estimated glomerular filtration rate of 41 mL/min/1.73 m2.
The patient was cognitively intact with no strokes. He had a clinical frailty score of 1 out of 4, a Society of Thoracic Surgeons score of 12.6, and a morbidity/mortality risk of 34.7%. An electrocardiogram revealed sinus bradycardia with inferior Q waves. An echocardiogram demonstrated preserved left ventricular function with an ejection fraction of 61%. His aortic valve mean pressure gradient was 38 mm Hg, with a valve area of 0.48 cm2, peak aortic jet velocity of 4.3 m/s, and a trileaflet valve. There was moderate aortic and mitral regurgitation.
A coronary angiogram demonstrated complete occlusions of the left main and right coronary arteries. The LIMA-to-LAD artery graft was patent and there were vein grafts to the PDA and the obtuse marginal artery. The SVG to LAD-D1 had failed.
Results of CT included a perimeter of 80.2 mm with an area of 495.5 mm2 (Figure 1), heavy sinotubular junction calcification (Figure 2), aortic root angulation of 46°, and left and right coronary heights of 11.6 mm and 15.2 mm, respectively.
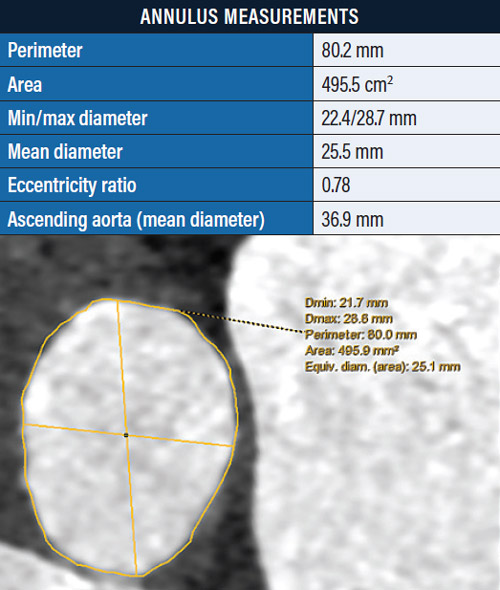
Figure 1. Annulus measurements.

Figure 2. Heavy calcification of the sinotubular junction.
The eccentricity ratio was 0.78. There was heavy calcification and significant tortuosity of the iliofemoral system (Figure 3).
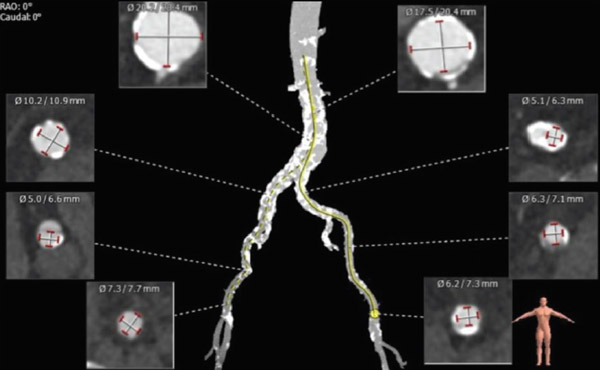
Figure 3. CT imaging showing heavy iliofemoral calcification and tortuosity.
Femoral artery access was considered acceptable. Subclavian access was suboptimal due to angulation and small size.
Treatment selection
The patient was discussed in our multidisciplinary meeting and was decided to be most suitable for transcatheter aortic valve implantation (TAVI) in view of the extreme risk for surgery. Despite the heavy iliofemoral disease, we believed right femoral access would be successful. Next, the choice of valve was considered. The sinotubular junction calcification was concerning for a balloon-expandable system, and because the iliac tortuosity and calcium would not be ideal for use of the Evolut system (Medtronic), we chose the Portico with FlexNav system (Abbott).
The eccentricity ratio of 0.78 was considered acceptable, with the recommendation being > 0.73.
Procedure description and results
The patient was admitted and elective TAVI was performed under conscious sedation with local anesthesia. Vascular access was achieved using ultrasound guidance via the right femoral artery with a preplaced Prostar XL closure device (Abbott) and a femoral venous balloon-tipped pacing wire. Contralateral access was gained in the left femoral artery.
Aortography was performed, and baseline aortic pressure gradients were obtained. A 14-F sheath was placed in the right femoral artery over a Confida guidewire (Medtronic). Balloon aortic valvuloplasty was performed with a 20- X 40-mm Nucleus-X balloon (B. Braun Interventional Systems, Inc.) with rapid pacing at 180 bpm. A 29-mm Portico valve was chosen. The valve was delivered via the 14-F integrated sheath with its hydrophilic coating.
The valve was very stable during deployment and was placed on the first attempt at a depth of 0 mm (Figure 4). Although a little higher than the target depth, the valve was very stable. No pacing was required during valve positioning. There was trivial paravalvular leak on aortography, and final valve deployment occurred. There was no new conduction disturbance.
The valve performed well, with an on-table echocardiogram revealing only trivial regurgitation. The mean hemodynamic pressure gradient was 6 mm Hg. A predischarge echocardiogram revealed excellent
valve function with trivial paravalvular leak. The patient was discharged from the hospital 3 days later without complication.
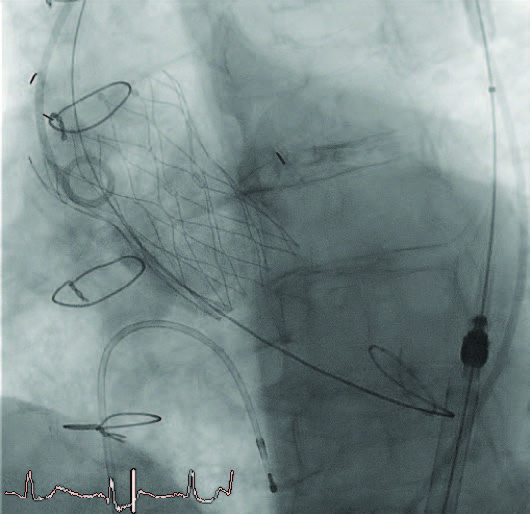
Figure 4. Valve position after implantation.
Discussion
A key advantage of the Portico system is its excellent trackability in tortuous vessels, including the iliac arteries and the aortic arch, which was particularly relevant in this case. The lower-profile integrated sheath allows treatment with the FlexNav delivery system in patients with previous femoral artery contraindications.
The FlexNav delivery system allows the valve to remain very stable during deployment. There is little need for pacing during deployment. The new stability layer has significantly improved the ease of placement with very little adjustment required. It is important to deploy slowly and wait several minutes after valve expansion to ensure that the position is stable and the valve can be finally deployed. The target depth is 2 to 5 mm below the annulus. The release mechanism of the FlexNav delivery system is easy to use and intuitive.
Coronary access is generally straightforward due to the intra-annular valve position and the large cell design. The system has a lower profile from the integrated sheath with hydrophilic coating. Blood pressure remains stable during deployment due to the early function of the Portico valve.
Predilatation of the valve is highly recommended prior to delivery system insertion. The balloon size should not exceed the minimum annulus diameter as derived by CT.
Conclusion
As demonstrated by this case, significant improvements have been made in the delivery system of the Portico valve. Primarily, the system is smaller with markedly improved stability during valve deployment. The flexibility in the iliofemoral system is excellent in more challenging anatomies. The next-generation Navitor with FlexNav TAVI system (Abbott), which is currently undergoing trial, may further add to the ease of use and effectiveness of this valve.
Antony Walton, MBBS, FRACP, FCSANZ
Associate Professor
Alfred Health
Melbourne, Victoria, Australia
Disclosures: Proctor for Abbott.
Portico With FlexNav TAVI System: Enhancing Innovative Technology to Optimize Patient Outcomes and Physician Experience
By Mike Morrissey
The original Portico transcatheter aortic valve implantation (TAVI) system (Abbott) comprises a transcatheter valve and a delivery system used to navigate and deploy the valve into position at the native aortic annulus. Throughout the initial clinical experience with the Portico system, several aspects were identified as major strengths. These included the low profile and overall flexibility of the delivery system; ease of tracking the system over the aortic arch; intra-annular valve positioning for hemodynamic stability during deployment; recapturability, repositionability, and retrievability of the valve; and large-cell geometry for easy coronary access postimplantation.
Over time, additional areas for improvement were identified as physicians expressed a need for sheathless vascular access, valve placement accuracy, and overall improvements in ease of use.
Latest-generation TAVI system
As a response to these customer requests, the FlexNav delivery system (Abbott; Figure 1) was developed to address improvement needs while maintaining the positive performance aspects of the original Portico system (see the Technology Design Goals Met By the FlexNav Delivery System sidebar). The FlexNav delivery system incorporates a stability layer to the catheter, reducing the amount of manipulation required at the access site and providing predictable, accurate, and stable implantation of the valve at the annulus. The addition of an integrated sheath onto the catheter allows easy, sheathless access into the vasculature and maintains a low insertion profile, allowing access into vessels as small as 5 mm for the small system and 5.5 mm for the large system.
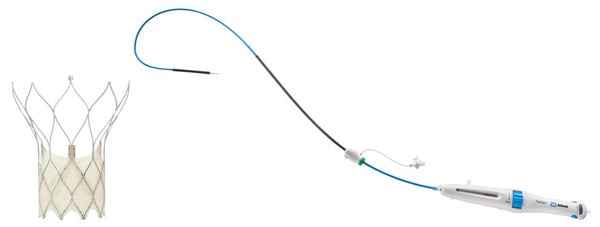
Figure 1. Portico with FlexNav TAVI system.
Additionally, the hydrophilic coating on the inserted length of the delivery system significantly reduces the surface frictional properties of the catheter and augments easy tracking through tortuous vessels. Lastly, ease-of-use improvements are accomplished through a redesigned handle with automation of the deployment lock mechanism, increased mechanical advantage (less force per turn), and the addition of a dedicated macro slide feature, all serving to simplify the user interface and provide a streamlined workflow. In total, the FlexNav delivery system represents a significant advancement and greatly simplifies and improves implantation of the Portico valve.
Improved placement accuracy and stability
Improvements in placement accuracy and stability were achieved with the addition of a stability layer to the main catheter assembly (Figure 2). This additional layer encapsulates the primary outer shaft, which contains the valve capsule and thus eliminates all motion of the delivery system at the vessel access site during deployment.
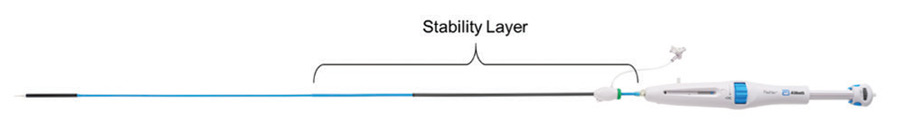
Figure 2. Stability layer.
By eliminating this motion, the user does not need to manipulate the delivery system at the access site to obtain valve placement accuracy because there is no tendency for the valve to “dive” into the left ventricle during implantation.
Low insertion profile
To maintain a low insertion profile, an integrated introducer sheath is incorporated into the delivery system proximal to the valve capsule (Figure 3).
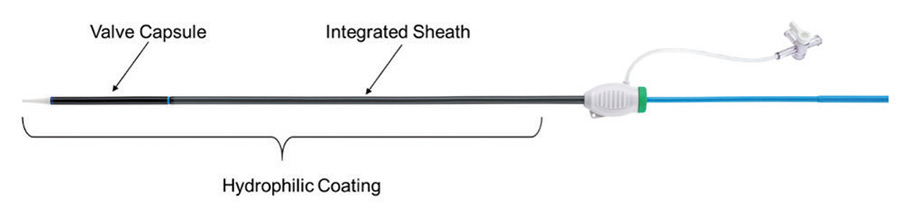
Figure 3. Integrated sheath and hydrophilic coating.
This integrated sheath has the same outer diameter as the delivery catheter, offering 14-F (6 mm) and 15-F (6.3 mm) equivalent sheath diameters for the two delivery system sizes (small size for 23- and 25-mm valves and large size for 27- and 29-mm valves) and allowing for insertion of the delivery system without the use of a separate introducer sheath. Additionally, the hydrophilic coating enhances tracking of the delivery system into the access vessel.
Ease of use
The last main area of focus for the FlexNav delivery system was an overall improvement in ease of use, which was largely achieved through a redesign of the control handle (Figure 4).
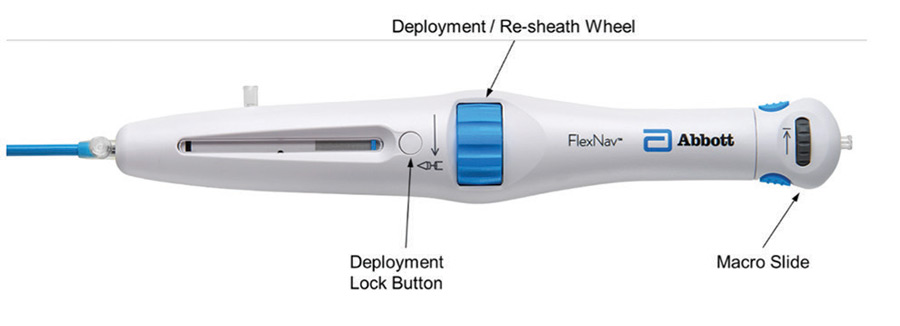
Figure 4. FlexNav control handle.
The primary deployment wheel mechanism was maintained; however, mechanical design improvements were made to give the deployment wheel an increased mechanical advantage and smoother action, providing a more consistent tactile feel during deployment and, if necessary, the ability to recapture the valve. In addition to the improved deployment mechanisms, the deployment lock button was redesigned to automatically engage during use, eliminating the need to manually engage the lock during device preparation and resheathing, thus reducing the overall number of steps to use the device. Lastly, a pullback handle (macro slide) was incorporated to clearly delineate the device closure mechanism from the deployment/recapture wheel and allow for atraumatic recapture of the distal tip of the catheter into the valve capsule after final valve release.
Apart from the redesigned control handle, additional ease-of-use improvements arise from the previously mentioned stability layer, which eliminates the need for manipulation to maintain implant position and reliable release of the valve from the delivery system on full deployment.
Deliverability
Excellent deliverability of the FlexNav system is achieved by leveraging the highly flexible shaft design of the original Portico delivery system as well as the Portico valve, which has less metal by design. This delivery system shaft was optimized for reliable resheathability without the need for significant metallic support structures, resulting in a very trackable system. For FlexNav, the addition of the integrated sheath and hydrophilic coating is intended to optimize deliverability even further. These features together are designed to easily traverse tortuous anatomy leading up to the aortic arch as well as horizontal aortas.
Summary
The Portico transcatheter valve system has been used to treat nearly 15,000 patients with aortic stenosis and continues to gain momentum in an increasingly crowded market.
Information contained herein for DISTRIBUTION outside of the U.S. ONLY.
Check the regulatory status of the device in areas where CE marking is not the regulation in force.
Page published on May 2020



