12 May 2020
Transfemoral TAVI in the horizontal aorta
Sponsored by Abbott
Real-world case report illustrating the benefits of the Portico with FlexNav TAVI system.
Portico with FlexNav TAVI system: enhancing innovative technology to optimize patient outcomes and physician experience
A case report by Francesco Bedogni, MD
Over the last decade, transcatheter aortic valve implantation (TAVI) has become an established treatment option for patients with severe aortic stenosis. Nowadays, transcatheter valves are approved across the entire spectrum of risk, from patients ineligible for surgery to those at low risk.1,2 However, extending TAVI indications to even lower-risk and younger patients necessitates safer and more accurate devices, even in those with unfavorable anatomy (ie, horizontal aorta, tortuosity, elliptic annuli). The FlexNav delivery system (Abbott), encompassing a reduced insertion profile, improved placement accuracy, and enhanced ease of use, aims to further improve procedural outcomes and expand the applicability of transfemoral TAVI to a greater proportion of patients. The following case report illustrates the utility of this newly designed delivery system.
Patient presentation
An 84-year-old woman presented to the outpatient clinic with a history of previous syncope and dyspnea for mild exertion due to severe calcific aortic stenosis (mean gradient, 45 mm Hg; effective orifice area, 0.76 cm2). Given her advanced age, frailty, and multiple comorbidities and a Society of Thoracic Surgeons predicted risk of mortality of 8%, the heart team considered the patient to be at high risk for surgery and recommended TAVI. Preprocedural multislice CT revealed a moderately calcified tricuspid aortic valve (perimeter, 61.4 mm; area, 293.6 mm2) with horizontal aortic root anatomy (58°; Figure 1) and favorable bilateral iliofemoral arteries with some degree of tortuosity and mild-to-moderate calcification at the aortic bifurcation (Figure 2).
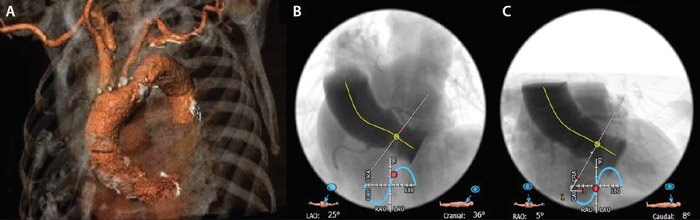
Figure 1. Preprocedural CT scan: three-dimensional rendering of aortic arch (A), left cranial (B), and right caudal (C) projections showing a 58° horizontal aorta.
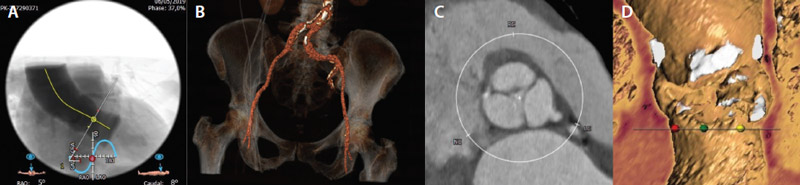
Figure 2. Preprocedural CT scan showing a 58° aortic angle (A), mild degree of tortuosity with moderate aortic bifurcation calcification (B), and a tricuspid aortic valve with moderate annular calcification (C, D).
Procedure description and results
TAVI was planned via a right transfemoral approach using the new FlexNav delivery system and a 23-mm Portico valve (Abbott). The procedure was performed under local anesthesia. After echo-guided right common femoral artery access (Figure 3) and preclosure with two Perclose ProGlide devices (Abbott), a 14-F sheath was inserted and a preshaped 0.035-inch stiff wire was placed into the left ventricle.
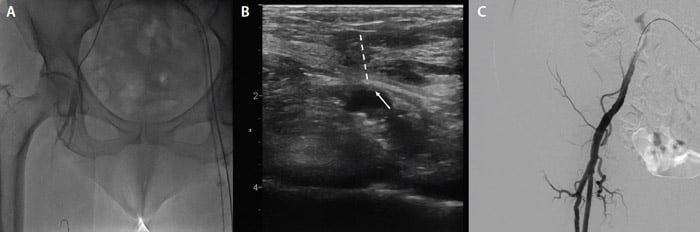
Figure 3. Basal iliofemoral angiogram (A), right femoral echo-guided puncture (dashed line indicates needle trajectory; arrow indicates puncture side) (B), and postprocedural iliofemoral angiogram (C).
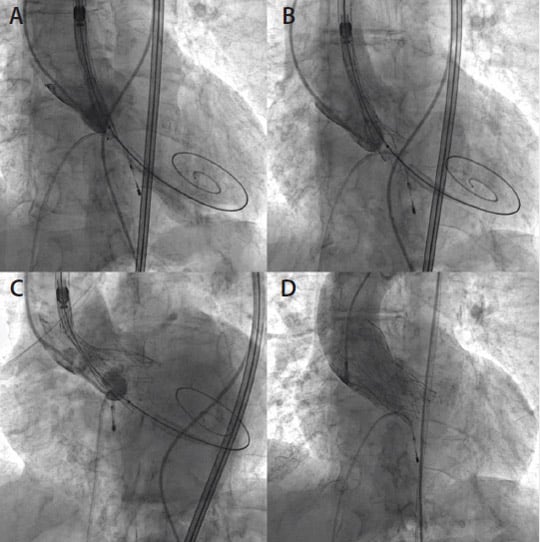
Aortic valve valvuloplasty with an 18-mm Cristal balloon (Balt) was performed. Afterward, the 14-F sheath was removed and the Portico valve was successfully advanced via the right femoral artery using the FlexNav delivery system and the integrated sheath. Slow and controlled release of the Portico device was performed, starting at the ideal depth under the annulus on the noncoronary cusp side. The increased placement accuracy of the FlexNav delivery system was instrumental in achieving optimal placement of the Portico device in the slightly horizontal aortic root, minimizing the need for repositioning and catheter manipulation (Figure 4).
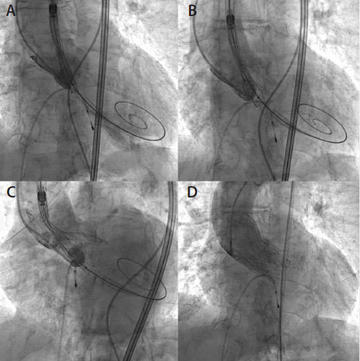
Figure 4. Portico valve positioning and deployment (A, B). The flexibility and adaptability of the Portico valve can be appreciated in difficult anatomy, including a horizontal aorta (C). Final result with trivial PVL (D).
Balloon postdilatation with a 20-mm Cristal balloon was performed to optimize the implant, achieving excellent angiographic and hemodynamic results. The postimplantation mean transvalvular aortic gradient measured by transthoracic echocardiography was satisfyingly low (6 mm Hg), with only a trivial amount of paravalvular leak (PVL). Access site hemostasis was successfully achieved using the preclosure sutures, and the right iliofemoral angiogram showed no dissection and normal antegrade contrast flow (Figure 3C).
Discussion
Many factors affect TAVI procedural outcomes, including the presence of calcifications, elliptic annuli, bicuspid aortic valve, vascular access, as well as a horizontal aorta, as this poses a degree of technical challenge for self-expanding devices. Indeed, extreme aortic angulation (AA) > 70° has been previously reported as an absolute exclusion criterion from a clinical trial of a self-expandable valve.3 Moreover, several studies identify AA as a factor influencing
the procedural outcome. Sherif et al found that a greater AA, measured using left ventriculography, was associated with a greater risk of PVL.4 Abramowitz et al showed that patients with an AA ≥ 48° who underwent TAVI with a self-expandable device, when compared with those who underwent TAVI with a balloon-expandable device, had an increased risk of valve embolization, need for a second valve, and postprocedural paravalvular regurgitation.5 The authors suggest that differences between self-expandable and balloon-expandable devices in stent frame length, stent deformation, radial force, and flexion control of the delivery system may be responsible for these findings.
In our case, despite a 58° AA, the improved placement accuracy of the FlexNav delivery system with the new stability layer and the enhanced flexibility and trackability allowed for optimal prosthesis positioning and deployment. There was no need for repositioning or the valve diving into left ventricular outflow tract, with an excellent procedural outcome.
Aside from the delivery system, the Portico valve has self-centering properties due to a reduced amount of metal and larger stent cells. Furthermore, due to the sheath integrated into the FlexNav system (14–15 F), the valve can be delivered without the requirement for a separate introducer, reducing the delivery sheath–to–femoral artery ratio, which has been found to be a strong predictor of major vascular complications.6
Conclusion
The redesigned FlexNav delivery system, with its improved valve placement accuracy and reduced insertion profile, may significantly improve daily practice by extending the possibility of transfemoral TAVI with the Portico valve to a greater proportion of patients. This novel technology has the potential to improve procedural outcomes by reducing the number of recaptures, risk of major vascular complications, malpositioning, or need for second valves.
References
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609-1620.
- US Food & Drug Administration. FDA expands indication for several transcatheter heart valves to patients at
- low risk for death or major complications associated with open-heart surgery. www.fda.gov/news-events/press-announcements/fda-expands-indication-several-transcatheter-heart-valves-patients-low-risk-death-or-major. Published August 16, 2019. Accessed September 9, 2019.
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790-1798.
- Sherif MA, Abdel-Wahab M, Stoecker B, et al. Anatomic and procedural predictors of paravalvular aortic regurgitation after implantation of the Medtronic CoreValve bioprosthesis. J Am Coll Cardiol. 2010;56:1623-1629.
- Abramowitz Y, Maeno Y, Chakravarty T, et al. Aortic angulation attenuates procedural success following self-expandable but not balloon-expandable TAVR. JACC Cardiovasc Interv. 2016;9:964-972.
- Hayashida K, Lefèvre T, Chevalier B, et al. Transfemoral aortic valve implantation: new criteria to predict vascular complications. JACC Cardiovasc Interv. 2011;4:851-858.
Francesco Bedogni, MD
Department of Clinical and Interventional Cardiology IRCCS Policlinico San Donato
Milan, Italy
[email protected]
Disclosures: Consultant to Abbott.
Portico With FlexNav TAVI System: Enhancing Innovative Technology to Optimize Patient Outcomes and Physician Experience
By Mike Morrissey
The original Portico transcatheter aortic valve implantation (TAVI) system (Abbott) comprises a transcatheter valve and a delivery system used to navigate and deploy the valve into position at the native aortic annulus. Throughout the initial clinical experience with the Portico system, several aspects were identified as major strengths. These included the low profile and overall flexibility of the delivery system; ease of tracking the system over the aortic arch; intra-annular valve positioning for hemodynamic stability during deployment; recapturability, repositionability, and retrievability of the valve; and large-cell geometry for easy coronary access postimplantation.
Over time, additional areas for improvement were identified as physicians expressed a need for sheathless vascular access, valve placement accuracy, and overall improvements in ease of use.
Latest-generation TAVI system
As a response to these customer requests, the FlexNav delivery system (Abbott; Figure 1) was developed to address improvement needs while maintaining the positive performance aspects of the original Portico system (see the Technology Design Goals Met By the FlexNav Delivery System sidebar). The FlexNav delivery system incorporates a stability layer to the catheter, reducing the amount of manipulation required at the access site and providing predictable, accurate, and stable implantation of the valve at the annulus. The addition of an integrated sheath onto the catheter allows easy, sheathless access into the vasculature and maintains a low insertion profile, allowing access into vessels as small as 5 mm for the small system and 5.5 mm for the large system.
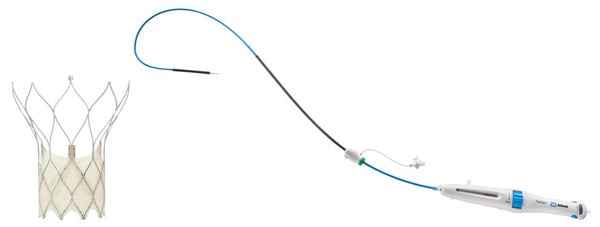
Figure 1. Portico with FlexNav TAVI system.
Additionally, the hydrophilic coating on the inserted length of the delivery system significantly reduces the surface frictional properties of the catheter and augments easy tracking through tortuous vessels. Lastly, ease-of-use improvements are accomplished through a redesigned handle with automation of the deployment lock mechanism, increased mechanical advantage (less force per turn), and the addition of a dedicated macro slide feature, all serving to simplify the user interface and provide a streamlined workflow. In total, the FlexNav delivery system represents a significant advancement and greatly simplifies and improves implantation of the Portico valve.
Improved placement accuracy and stability
Improvements in placement accuracy and stability were achieved with the addition of a stability layer to the main catheter assembly (Figure 2). This additional layer encapsulates the primary outer shaft, which contains the valve capsule and thus eliminates all motion of the delivery system at the vessel access site during deployment.
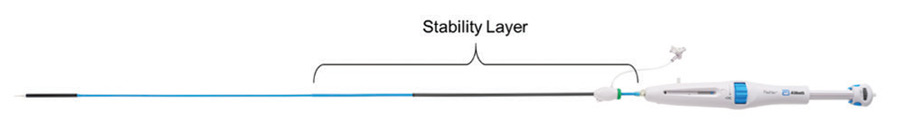
Figure 2. Stability layer.
By eliminating this motion, the user does not need to manipulate the delivery system at the access site to obtain valve placement accuracy because there is no tendency for the valve to “dive” into the left ventricle during implantation.
Low insertion profile
To maintain a low insertion profile, an integrated introducer sheath is incorporated into the delivery system proximal to the valve capsule (Figure 3).
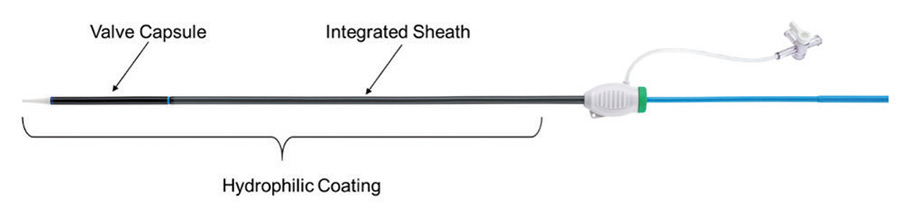
Figure 3. Integrated sheath and hydrophilic coating.
This integrated sheath has the same outer diameter as the delivery catheter, offering 14-F (6 mm) and 15-F (6.3 mm) equivalent sheath diameters for the two delivery system sizes (small size for 23- and 25-mm valves and large size for 27- and 29-mm valves) and allowing for insertion of the delivery system without the use of a separate introducer sheath. Additionally, the hydrophilic coating enhances tracking of the delivery system into the access vessel.
Ease of use
The last main area of focus for the FlexNav delivery system was an overall improvement in ease of use, which was largely achieved through a redesign of the control handle (Figure 4).
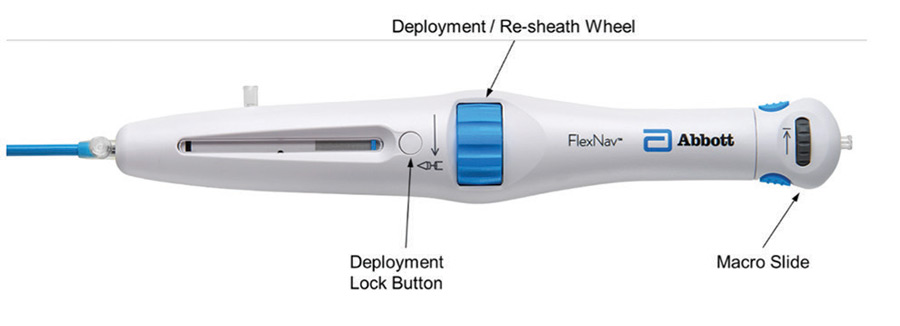
Figure 4. FlexNav control handle.
The primary deployment wheel mechanism was maintained; however, mechanical design improvements were made to give the deployment wheel an increased mechanical advantage and smoother action, providing a more consistent tactile feel during deployment and, if necessary, the ability to recapture the valve. In addition to the improved deployment mechanisms, the deployment lock button was redesigned to automatically engage during use, eliminating the need to manually engage the lock during device preparation and resheathing, thus reducing the overall number of steps to use the device. Lastly, a pullback handle (macro slide) was incorporated to clearly delineate the device closure mechanism from the deployment/recapture wheel and allow for atraumatic recapture of the distal tip of the catheter into the valve capsule after final valve release.
Apart from the redesigned control handle, additional ease-of-use improvements arise from the previously mentioned stability layer, which eliminates the need for manipulation to maintain implant position and reliable release of the valve from the delivery system on full deployment.
Deliverability
Excellent deliverability of the FlexNav system is achieved by leveraging the highly flexible shaft design of the original Portico delivery system as well as the Portico valve, which has less metal by design. This delivery system shaft was optimized for reliable resheathability without the need for significant metallic support structures, resulting in a very trackable system. For FlexNav, the addition of the integrated sheath and hydrophilic coating is intended to optimize deliverability even further. These features together are designed to easily traverse tortuous anatomy leading up to the aortic arch as well as horizontal aortas.
Summary
The Portico transcatheter valve system has been used to treat nearly 15,000 patients with aortic stenosis and continues to gain momentum in an increasingly crowded market.
Information contained herein for DISTRIBUTION outside of the U.S. ONLY.
Check the regulatory status of the device in areas where CE marking is not the regulation in force.
Page published on May 2020