03 Dec 2021
Real-world outcomes with the PASCAL Platform in TR
Sponsored by Edwards Lifesciences
The PASCAL platform, comprising the PASCAL and PASCAL Ace implants, received its CE mark for TR in May 2020 and has been shown to be an effective and safe treatment option for patients with TR.3,4,6 Professor Jörg Hausleiter and Dr Mirjam Wild now describe their single-centre experience from the LMU University Hospital in Munich, a high-volume centre with extensive experience in edge-to-edge repair, showing similar outcomes with the PASCAL and PASCAL Ace implants in patients with TR.
Professor Dr Jörg Hausleiter is Professor of Medicine and the Deputy Clinic Director at the Ludwig-Maximilians Universität (LMU) in Munich. Professor Hausleiter has been Principal Investigator in many clinical trials, including TRICuspid, bRIGHT, MiCLASP, TRILUMINATE and CLASP IID/IIF.
Dr Mirjam Wild is a Research Fellow in the Valvular Heart Team at LMU University Hospital in Munich and recently completed a fellowship in the Structural Heart Team at Bern University Hospital, Switzerland. Dr Wild’s research interests include new devices and treatment strategies for percutaneous mitral and tricuspid valve interventions, and cardiovascular imaging
The PASCAL platform has a number of specific features that are key to the successful treatment of patients with TR,1,3 including a high degree of flexibility, which enhances manoeuvrability when accessing the tricuspid valve.8 Independent grasping is also well established with the PASCAL platform and, along with the central spacer and broad paddles, allows the user to bridge large coaptation gaps with minimal leakage.1,8
The nitinol construction of the PASCAL platform provides flexibility for the implants to move with the leaflets during the cardiac cycle, reducing stress on the leaflets.1,8
While many of the key features of the PASCAL platform are shared between the PASCAL and PASCAL Ace implants, the PASCAL Ace implant has a narrower central spacer and profile, which helps with navigation of the device through the subvalvular apparatus.8 This is especially useful in the tricuspid valve, which has a dense network of thin, fragile chordae.9 In general, the benefit of having multiple implants with different features is to broaden the spectrum of patients who can be treated, leading to a more tailored approach in which the individual patient’s anatomy can be considered.
The big advantage of having multiple implants with different features is that you broaden the spectrum of patients who can be treated. A more tailored approach can be used which takes account of the patient’s anatomy
Professor Jörg Hausleiter
Munich single-centre experience
At the LMU University Hospital in Munich, we routinely treat patients with TR using the PASCAL platform, either with the PASCAL or the PASCAL Ace implant. We have conducted a prospective data collection and analysis of all patients treated with the PASCAL Ace implant since October 2020, and a descriptive analysis and comparison with a comparable patient cohort treated with the PASCAL implant. In this analysis, 78 patients with TR were treated with the PASCAL platform, including 50 with the PASCAL implant and 28 with the PASCAL Ace implant. There was a high technical success rate with both implants (ranging from 95–100%) and no significant difference in the mean number of devices implanted per patient (PASCAL implant 1.8; PASCAL Ace implant 1.5).7
There was a high technical success rate with both implants
Professor Jörg Hausleiter
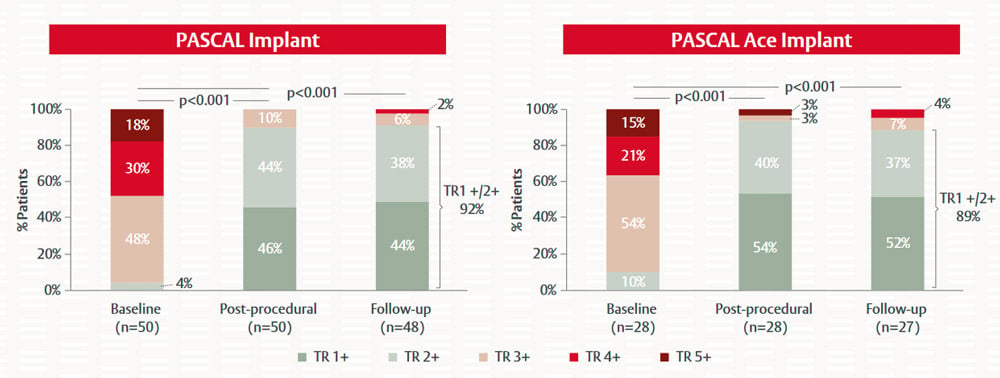
Figure 5. Impact of PASCAL and PASCAL Ace implantation on TR severity in patients with severe TR or greater7
Wild M. EuroPCR 2021. Reproduced with permission
TR severity was significantly reduced in patients treated with the PASCAL and the PASCAL Ace implants (Figure 5). While over 90% of patients had TR severity ≥3+ at baseline, mild-to-moderate TR (≤2+) was achieved in 92% of patients receiving the PASCAL implant and in 89% of patients receiving the PASCAL Ace implant at the 30-day follow up. Similarly, patients demonstrated a significant improvement in heart failure symptoms and NYHA functional class with both the PASCAL and PASCAL Ace implants (Figure 6). There were no device-related procedural complications with the two implants and only one patient had a single-leaflet device attachment.7
With both [PASCAL] implants, at least 90% of patients had moderate or less TR immediately after the procedure and at 30-day follow-up, indicating very good efficacy
Professor Jörg Hausleiter
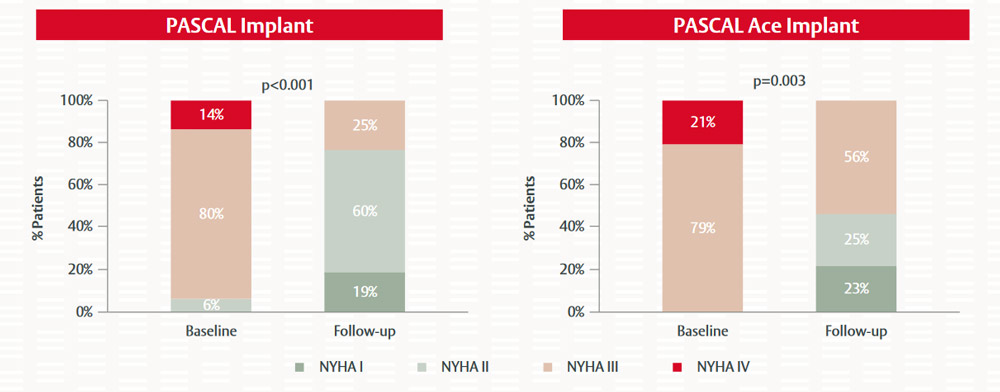
Figure 6. Impact of PASCAL and PASCAL Ace implantation on NYHA class in patients with severe TR or greater7
Wild M. EuroPCR 2021. Reproduced with permission
Overall, we found no significant difference in the extent to which the PASCAL and PASCAL Ace implants reduced TR severity and improved clinical outcomes in patients with severe TR.7
The safety profile with the PASCAL platform was very good. There were no relevant procedural complications that were device-related
Professor Jörg Hausleiter
Conclusion
Our single-centre experience at the LMU in Munich adds further evidence of the efficacy and safety of the PASCAL platform in treating patients with severe or greater TR.7 With the availability of different devices for TTVr, including the PASCAL and PASCAL Ace implants that make up the PASCAL platform, there is an opportunity to adapt TTVr treatments to individual patients based on their specific anatomies. However, before clear conclusions can be drawn in this regard, additional studies are required to determine which patients are most suitable for each treatment approach.
See what we’re making possible
References :
- Fam NP, Braun D, von Bardeleben RS et al. Compassionate use of the PASCAL Transcatheter Valve Repair System for severe tricuspid regurgitation: A multicenter, observational, first-in-human experience. JACC: Cardiovascular Interventions.2019; 12: 2488–95.
- Lurz P, Fam N, Kitamura M et al. Six-month results of the PASCAL transcatheter valve repair system for severe tricuspid regurgitation: A multicentre, observational, first-in-human experience. PCR e-Course: Interventions for valvular disease 2020, Euro20A-POS487,p.786
- Kitamura M, Fam NP, Braun D et al. 12-Month outcomes of transcatheter tricuspid valve repair with the PASCAL system for severe tricuspid regurgitation. Catheter Cardiovasc Interv. 2021; 97: 1281–89.
- Eleid M. Transcatheter tricuspid valve repair: CLASP TR early feasibility study six-month results. EuroPCR 2021.
- Kodali S, Hahn RT, Eleid MF et al. Feasibility study of the transcatheter valve repair system for severe tricuspid regurgitation. J Am Coll Cardiol. 2021; 77: 345–56.
- Edwards Lifesciences. Edwards PASCAL Transcatheter Valve Repair System receives European approval for tricuspid repair. May 2020.
- Wild M. Early experience with a new leaflet repair device for mitral and tricuspid regurgitation. EuroPCR 2021, and personal communication.
- Edwards Lifesciences. PASCAL Ace implant component design enhancement. Internal data on file. 2020.
- Pozzoli A, Zuber M, Reisman M et al. Comparative anatomy of mitral and tricuspid valve: What can the interventionlist learn from the surgeon. Front Cardiovasc Med. 2018; 5: 1–9.
Page published on December 2021.