31 Oct 2017
Edwards Lifesciences: SAPIEN XT™ Valve and SAPIEN 3™ Valve
Transcatheter aortic valve devices
The Edwards SAPIEN XT™ Valve and SAPIEN 3™ Valves are built upon the proven benefits of the SAPIEN valve family. Designed to minimize paravalvular leak while achieving an ultra-low delivery profile.
Edwards, Edwards Lifesciences, Edwards SAPIEN, Edwards SAPIEN XT, Edwards SAPIEN 3, SAPIEN, SAPIEN XT and SAPIEN 3 are trademarks of Edwards Lifesciences Corporation.
Images courtesy of Edwards Lifesciences LLC, Irvine, CA
Table of content
- Edwards SAPIEN XT™ Valve
- Edwards SAPIEN 3™ Valve
SAPIEN XT™ Valve introduction
The Edwards SAPIEN XT transcatheter heart valve is approved for delivery with the NovaFlex+ system and Ascendra+ system in 23 mm, 26 mm and 29 mm valve sizes.
- Balloon-expandable valve design for predictable deployment
- Low profile 16F access*
*For the 23 mm Edwards SAPIEN XT valve via the transfemoral procedure.
Approved for valve-in-valve procedures
- In failed surgical aortic bioprostheses for patients at high or greater surgical risk
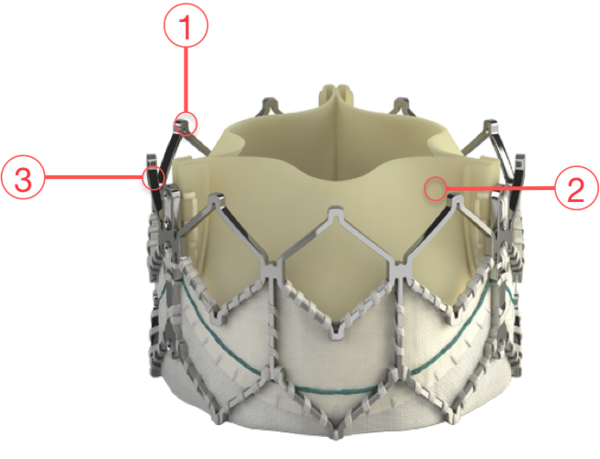
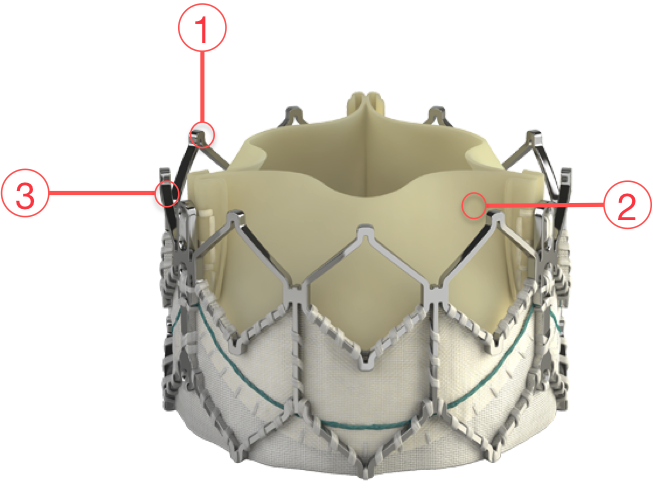
Figure 77. Edwards SAPIEN XT Transcatheter Heart Valve
1 - CIRCULARITY
- Promotes uniform leaflet coaptation for valve durability and optimal haemodynamic performance
- Circular valve design with high radial strength promotes uniform leaflet coaptation
- Predictable circular valve deployment through balloon-expandable inflation
2 - PROVEN VALVE TISSUE
- Edwards bovine pericardial tissue valves are the gold standard in surgical valve replacement
- Leaflet design and matching enhance stress distribution
- The Carpentier-Edwards ThermaFix process minimises the risk of calcification
3 - FRAME HEIGHT
- Designed to respect the cardiac anatomy and minimise the risk of conduction system interference
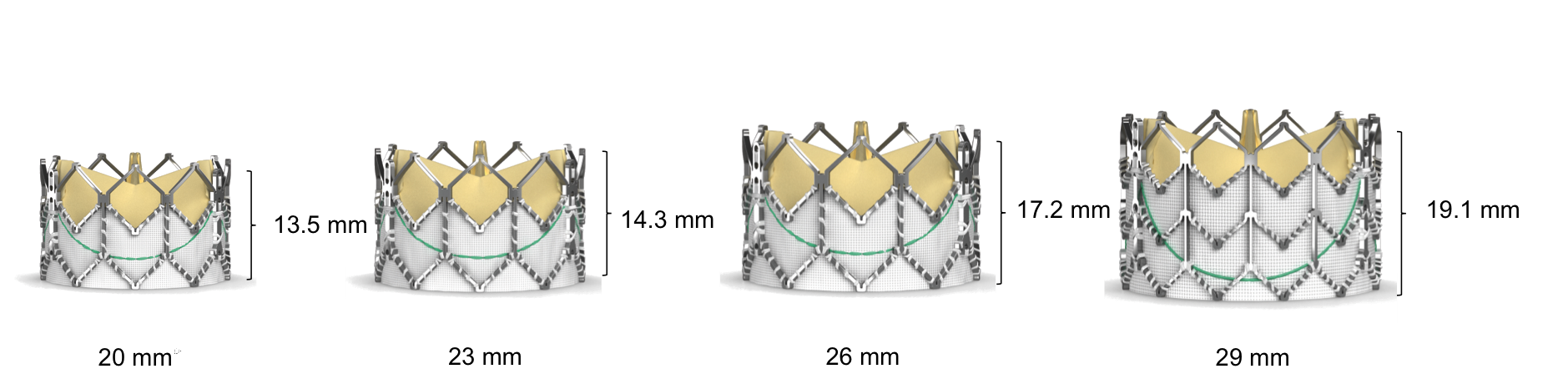
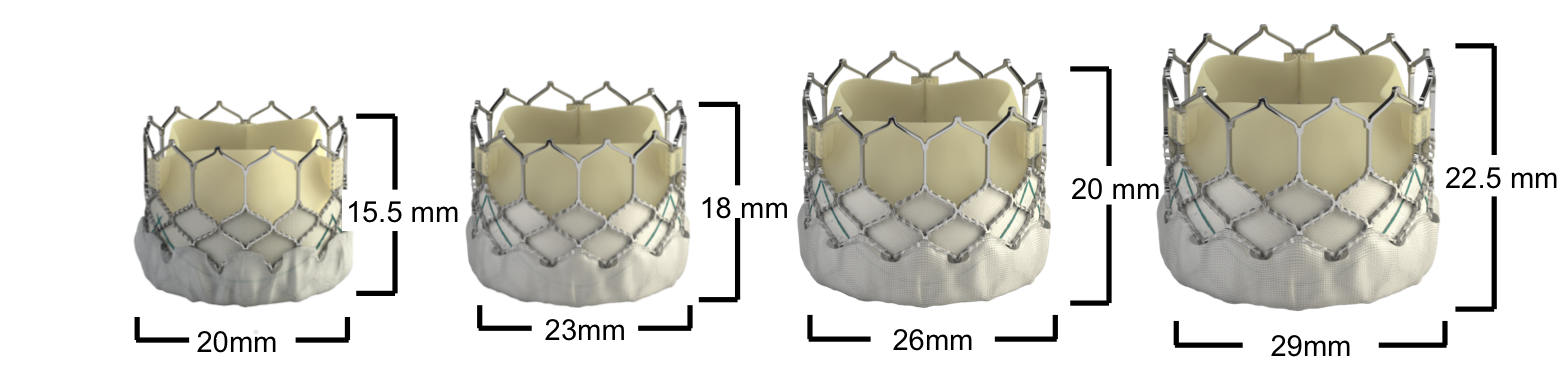
Figure 78. Edwards SAPIEN XT Transcatheter Heart Valve dimensions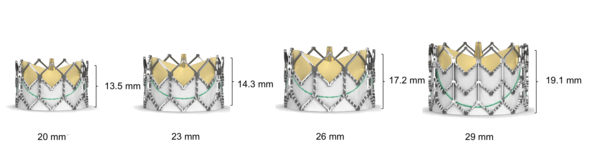
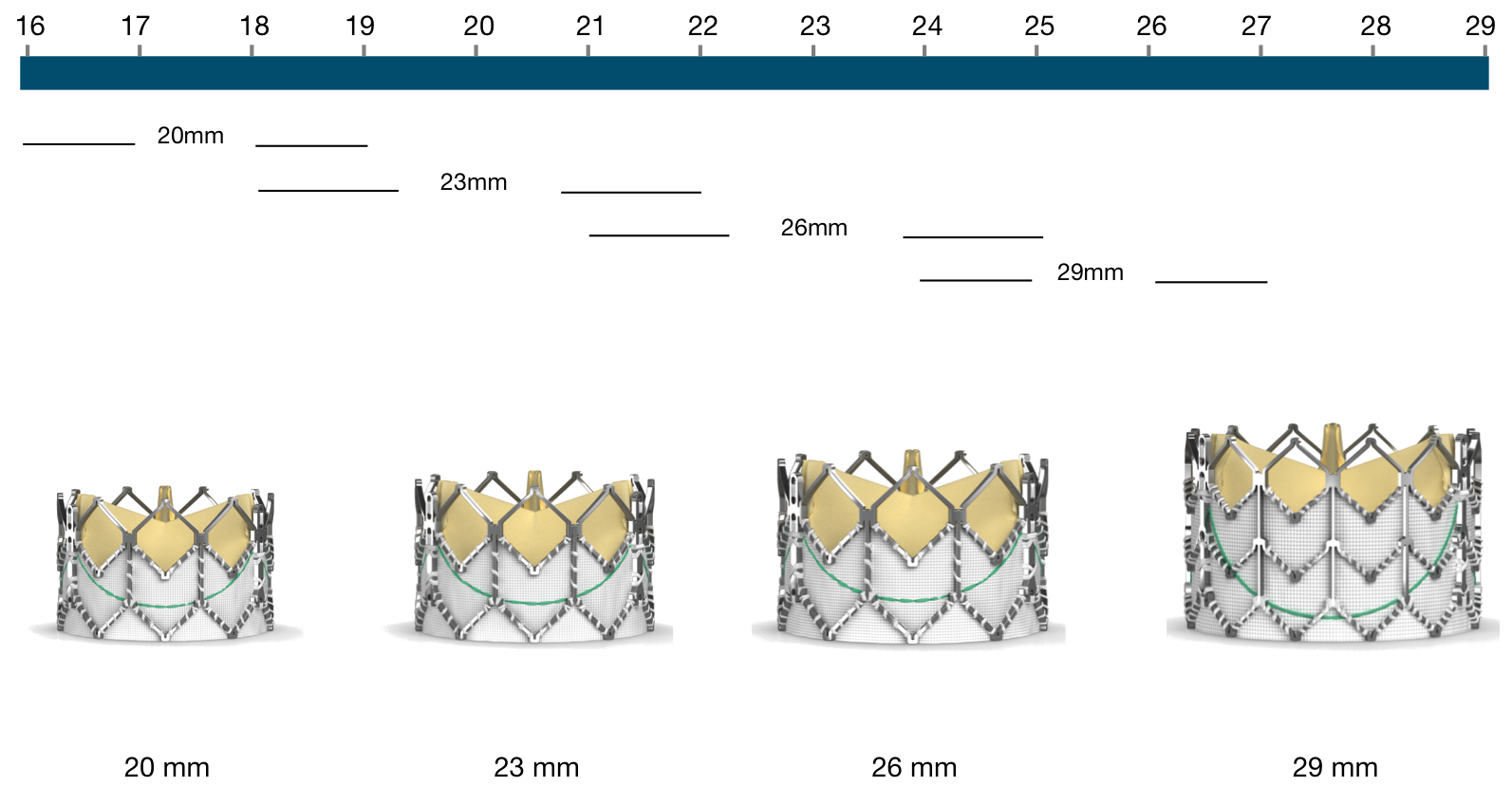
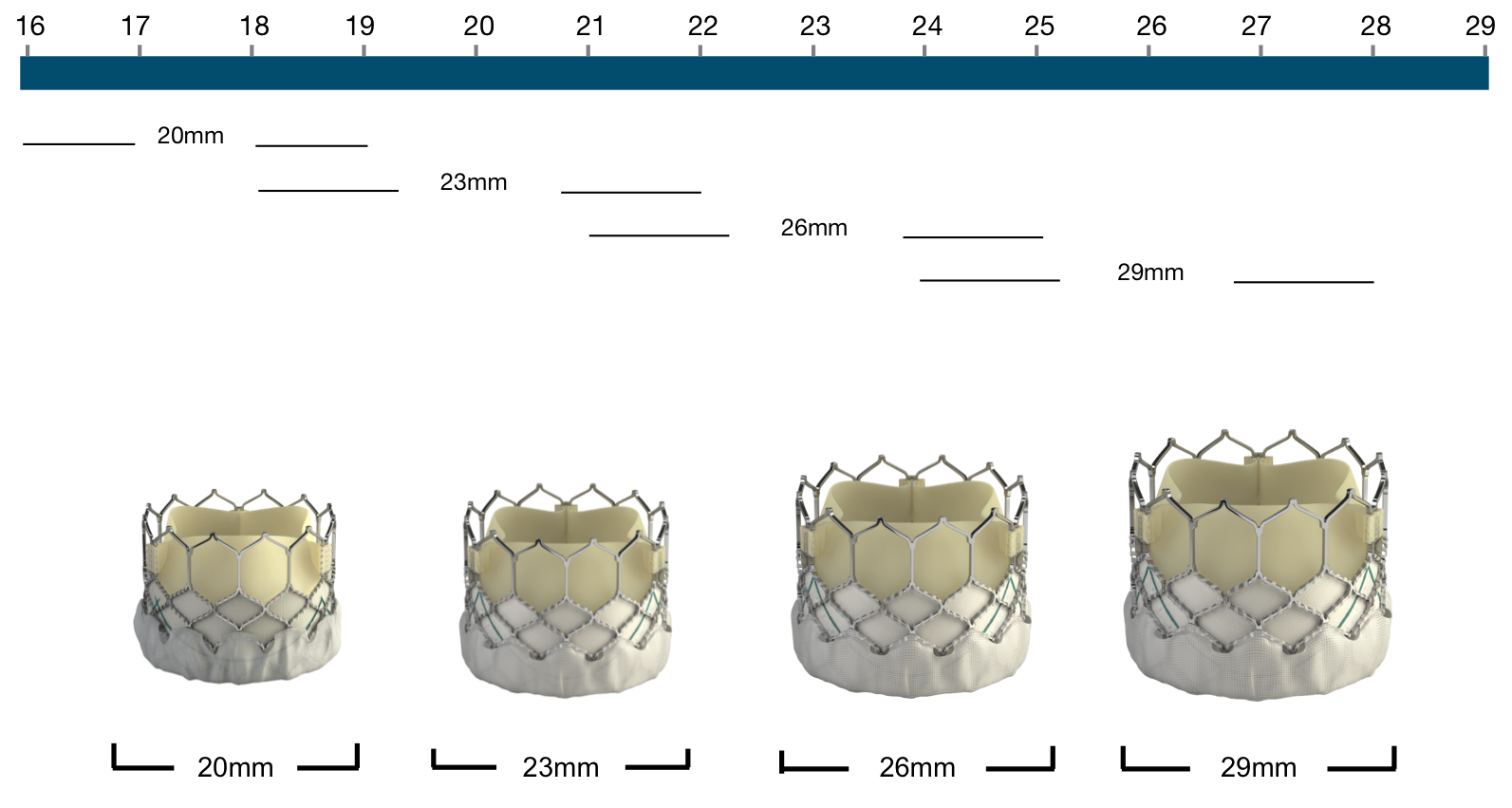
Figure 79. Edwards SAPIEN XT Transcatheter Heart Valve native annulus diameter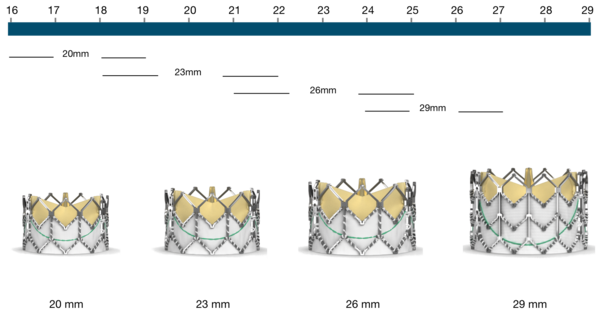
Edwards SAPIEN XT | Native annulus diameter* |
|---|---|
20 mm | 16-19 mm |
23 mm | 18-22 mm |
26 mm | 21-25 mm |
29 mm | 24-27 mm |
*as assessed by TEE
Edwards SAPIEN XT™ Valve animation
Edwards SAPIEN XT™ Transcatheter Heart Valve with the Nofaflex+ Transfemoral system
Edwards SAPIEN XT™ Transcatheter Heart Valve with the Ascendra+ Delivery system (Transaortic)
Edwards SAPIEN XT™ Transcatheter Heart Valve with the Ascendra+ Delivery system (Transapical)
General characteristics:
Design | Balloon-expandable |
Frame | Cobalt chromium |
Leaflets | Bovine pericardial tissue leaflets |
Valve size (mm) | 20 mm, 23 mm, 26 mm, 29 mm |
Delivery system diameter | - Transfemoral (Figure 80): |
Implantation access | Transfemoral, Transapical, Transaortic |
Repositionable | No |
Retrievable after being fully deployed | No |
Additional cuff to reduce AR | No |
Anchoring mechanism | - |
Tactile feedback during deployment | - |
Large stent cell design | Yes |
Markers to facilitate deployment | Radiopaque frame |
Motorised delivery system | - |
Anticalcification technology | Yes |
CE mark status: Yes - 02/03/2010
- Edwards SAPIEN XT: Angiograms
- Edwards SAPIEN XT: Phantom model implantation
- Edwards SAPIEN XT: 3D rotational animation
Click here for more information
SAPIEN 3™ Valve introduction
The Edwards SAPIEN 3™ Valve is built upon the proven benefits of the SAPIEN valve family. Designed to minimize paravalvular leak while achieving an ultra-low delivery profile, while maintaining radial strength for circularity. The expanded annulus size coverage and reduced minimum access vessel diameters.
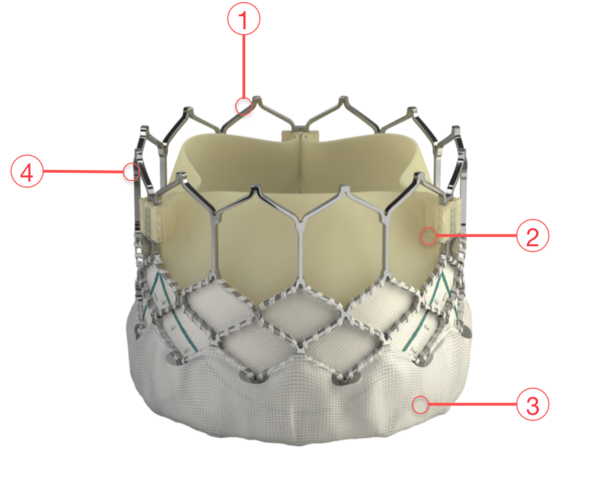
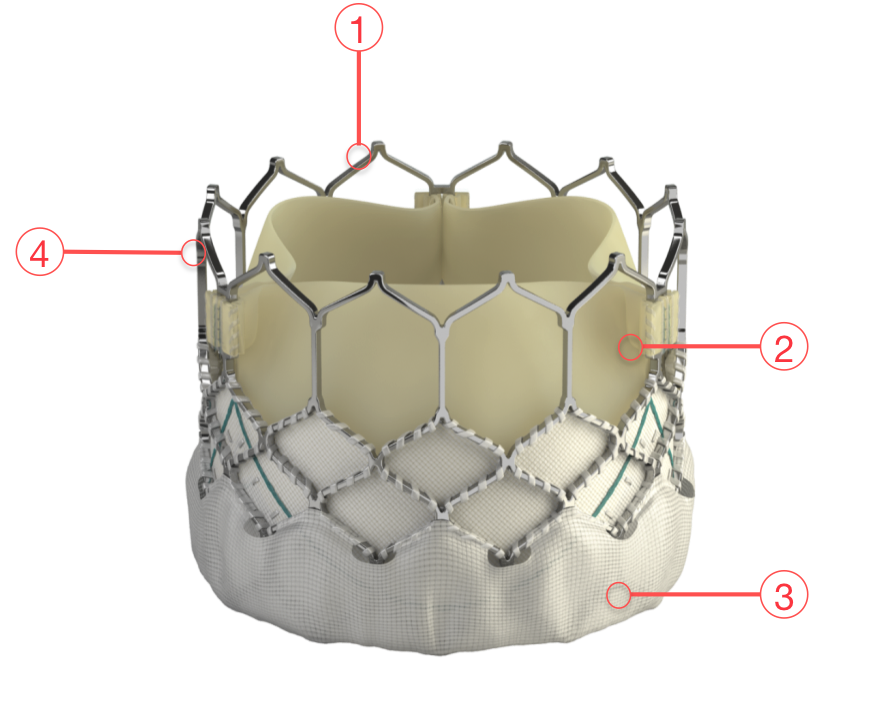
Figure 71. Edwards SAPIEN 3 Transcatheter Heart Valve
1 - FRAME DESIGN
Enhanced frame geometry for ultra-low delivery profile High radial strength for circularity and optimal haemodynamics
2 - BOVINE PERICARDIAL TISSUE
Optimised leaflet shape Carpentier-Edwards ThermaFix process for anticalcification
3 - OUTER SKIRT
Designed to reduce paravalvular leak
4 - LOW FRAME HEIGHT
Respects the cardiac anatomy
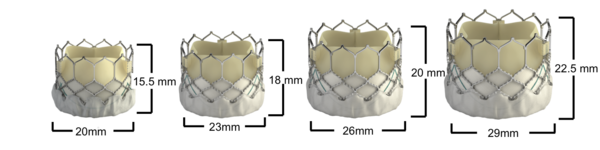
Figure 72. Edwards SAPIEN 3 Transcatheter Heart Valve dimensions
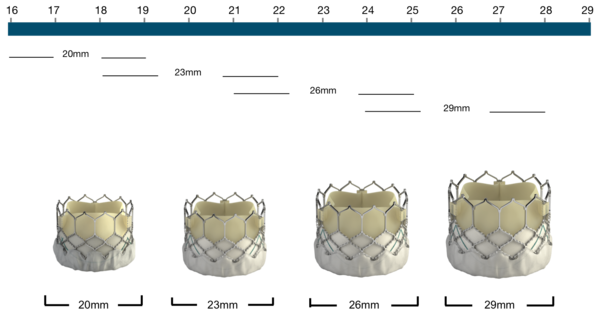
Figure 73. Edwards SAPIEN 3 Transcatheter Heart Valve native annulus diameter
Edwards SAPIEN 3 | Native annulus diameter* |
|---|---|
20 mm | 16-19 mm |
23 mm | 18-22 mm |
26 mm | 21-25 mm |
29 mm | 24-28 mm |
Edwards SAPIEN 3 | Native annulus area | Area-derived diameter |
|---|---|---|
20 mm | 273-345 mm2 | 10.6-21.0 mm |
23 mm | 338-430 mm2 | 20.7-23.4 mm |
26 mm | 430-546 mm2 | 23.4-26.4 mm |
29 mm | 540-680 mm2 | 26.2-29.5 mm |
General characteristics:
Design | Balloon-expandable (Figure 75) |
Frame | Cobalt chromium |
Leaflets | Bovine pericardial tissue leaflets |
Valve size (mm) | 20*mm, 23 mm, 26 mm, 29 mm |
Delivery system diameter | - Transfemoral (Figure 74): |
Implantation access | Transfemoral, Transapical, Transaortic |
Repositionable | No |
Retrievable after being fully deployed | No |
Additional cuff to reduce AR | Yes - outer skirt |
Anchoring mechanism | - |
Tactile feedback during deployment | - |
Large stent cell design | Yes |
Markers to facilitate deployment | Yes |
Motorised delivery system | No |
Anticalcification technology | Yes |
CE mark status: Yes - 27/01/2014
Special features:
- *20mm SAPIEN 3 valve is currently under clinical investigation and not available for commercial use