30 Oct 2017
Medtronic: CoreValve® Evolut™ R, CoreValve® Evolut™ and CoreValve®
Transcatheter aortic valve devices
The Medtronic CoreValve® Evolut™ R, CoreValve® Evolut™ and CoreValve™ system. The CoreValve Platform was designed specifically to meet the clinical needs of your TAVI patients.
The CoreValve, Evolut R and Evolut Pro are registered trademarks of Medtronic, Inc.
Table of content
- Medtronic CoreValve® Evolut™ R
- Medtronic CoreValve® Evolut™
- Medtronic CoreValve®
CoreValve® Evolut™ R Introduction
The new recapturable, self-expanding CoreValve™ Evolut™ R System for transcatheter aortic valve implantation (TAVI) in severe aortic stenosis patients who are at high or extreme risk for surgery. The CoreValve™ Evolut™ R recapturable and repositionable heart valve improve positioning accuracy and control during deployment.
Untreated, aortic valve stenosis can lead to serious heart problems including heart failure and even death. Designed to treat patients with aortic stenosis, a condition where the aortic valve narrows thereby limiting blood flow from the aorta to the rest of the body, the CoreValve Evolut R System is built on the proven foundation and procedural success of the CoreValve System, which has been implanted in more than 75,000 patients in 60 countries.
CoreValve Evolut R TAVI system has received CE approval to expand the indication to include patients who are at high or greater risk for surgical aortic valve replacement OR are ≥75 years of age and at intermediate risk for surgical AVR (Society of Thoracic Surgeons operative risk score ≥4% or with an estimated hospital mortality ≥4% as assessed by the heart team).
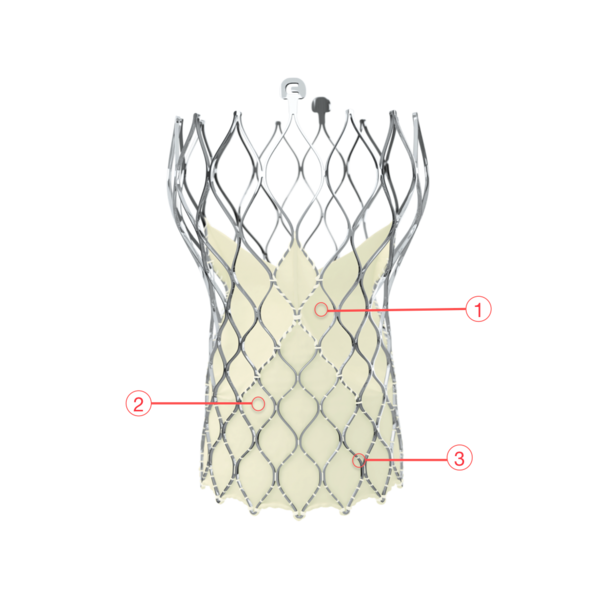
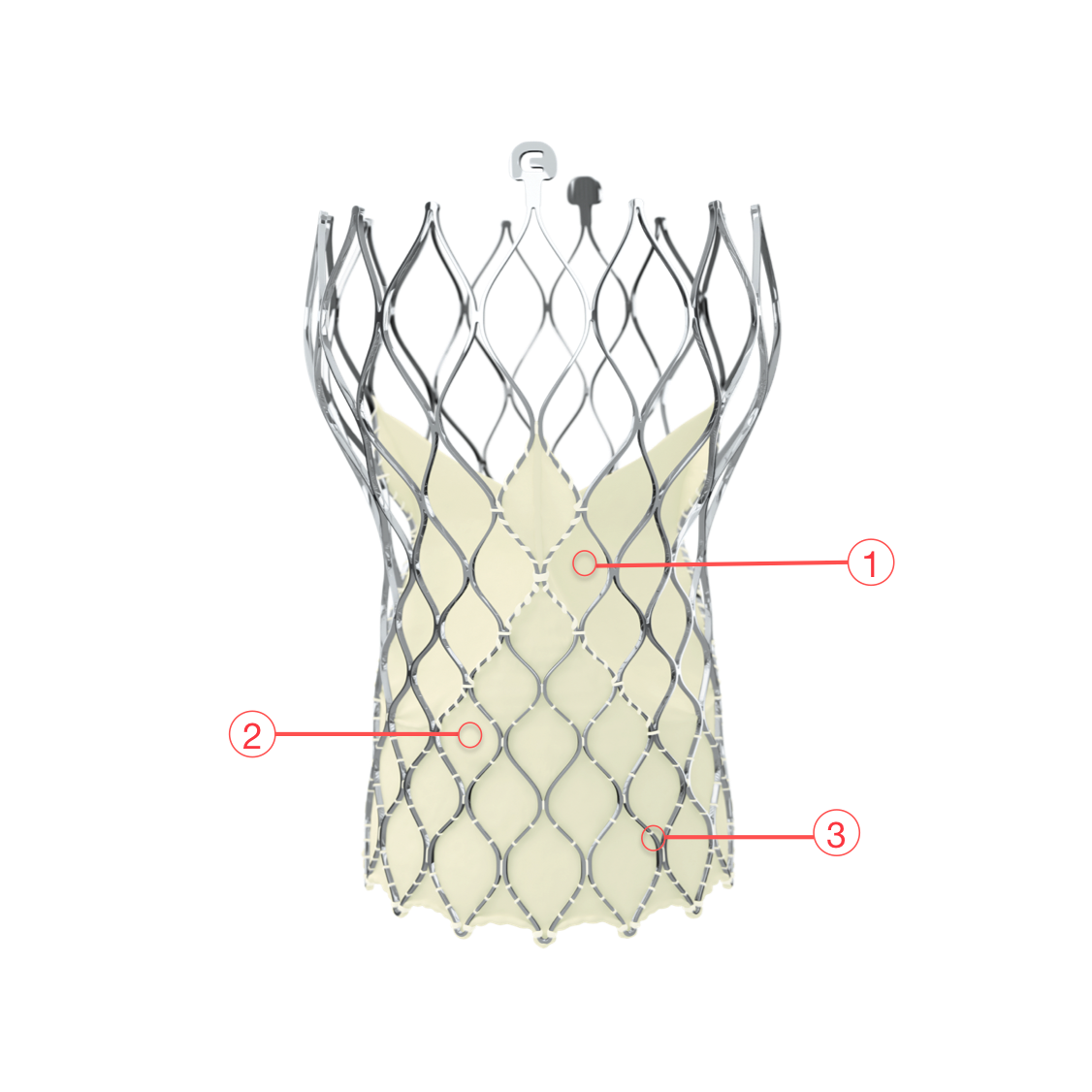
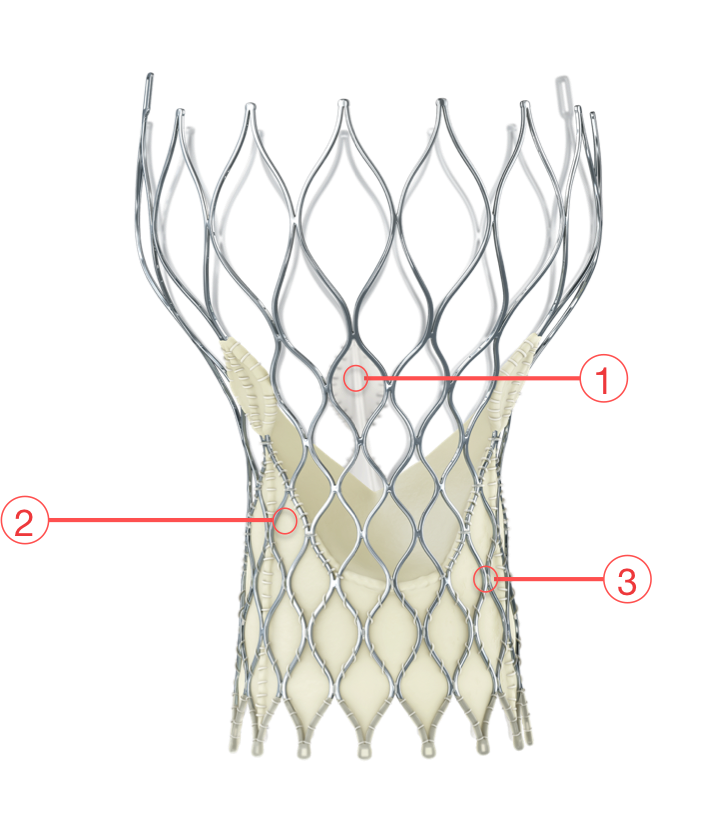
Figure 110. Medtronic CoreValve Evolut R transcatheter aortic valve
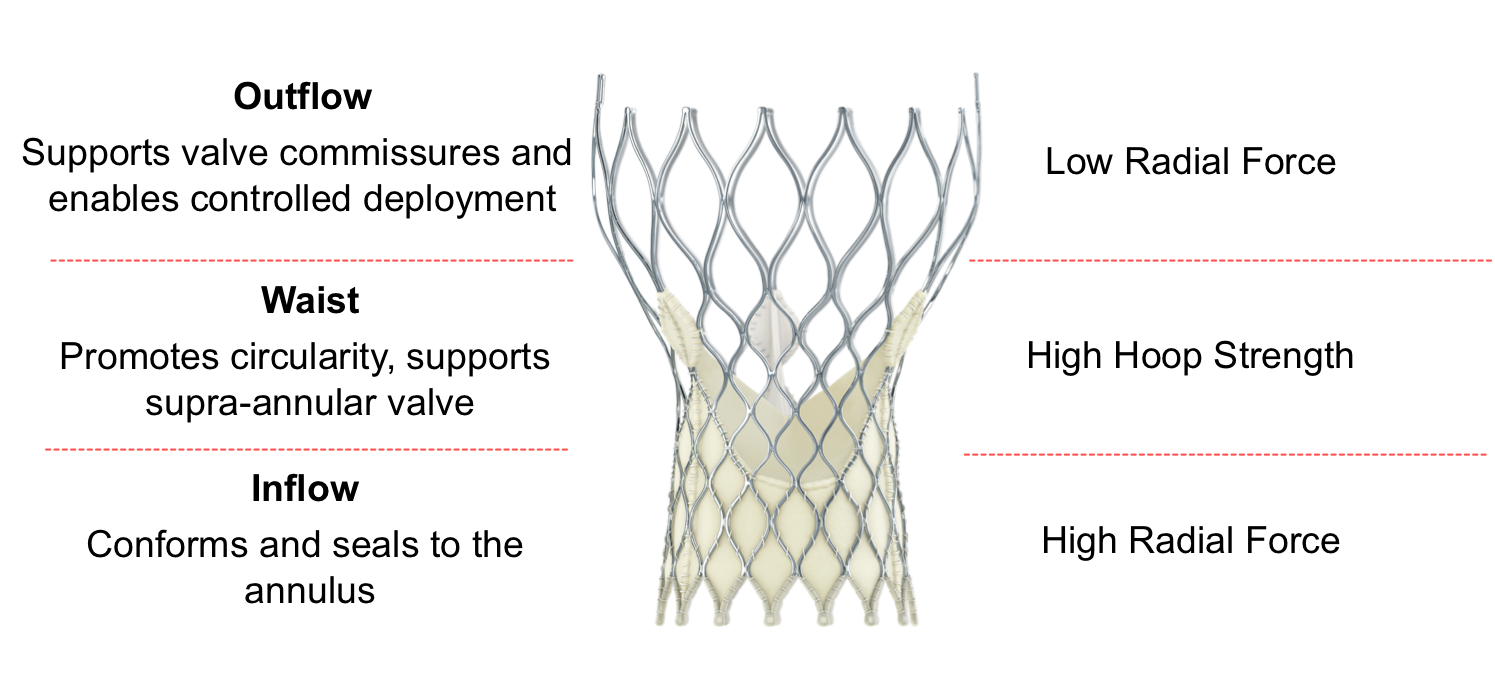
1 - SUPRA-ANNULAR VALVE DESIGN
- Conforms to the non-circular annulus while maintaining circularity at the level of coaptation
2 - PORCINE PERICARDIAL TISSUE
- Strong tissue, yet thin for low-profile delivery
3 - SELF-EXPANDING NITINOL FRAME
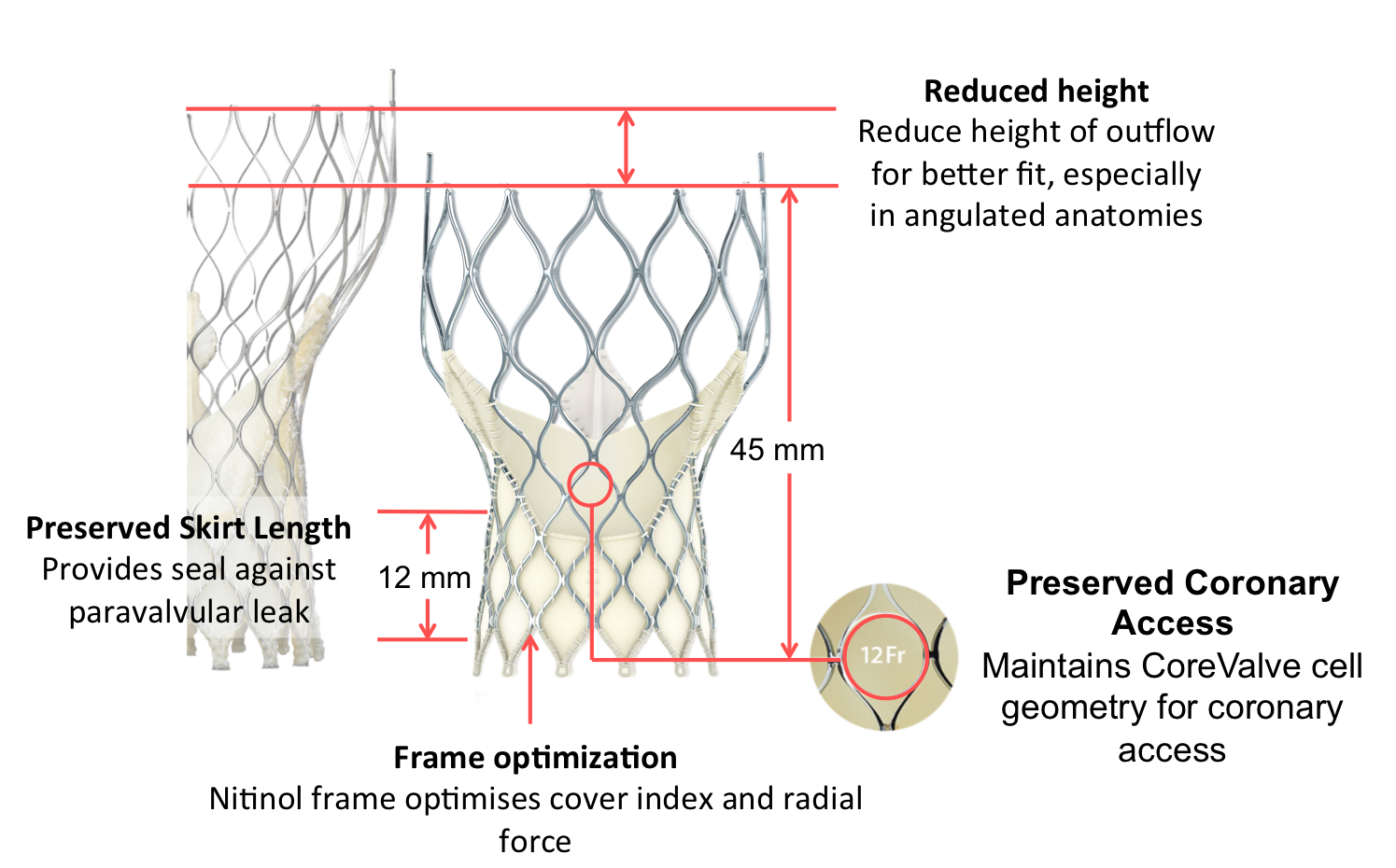
- Optimized cover index for annulus diameter range and implant depth
- Improved sealing and PVL performance with consistent radial force across the annulus diameter range
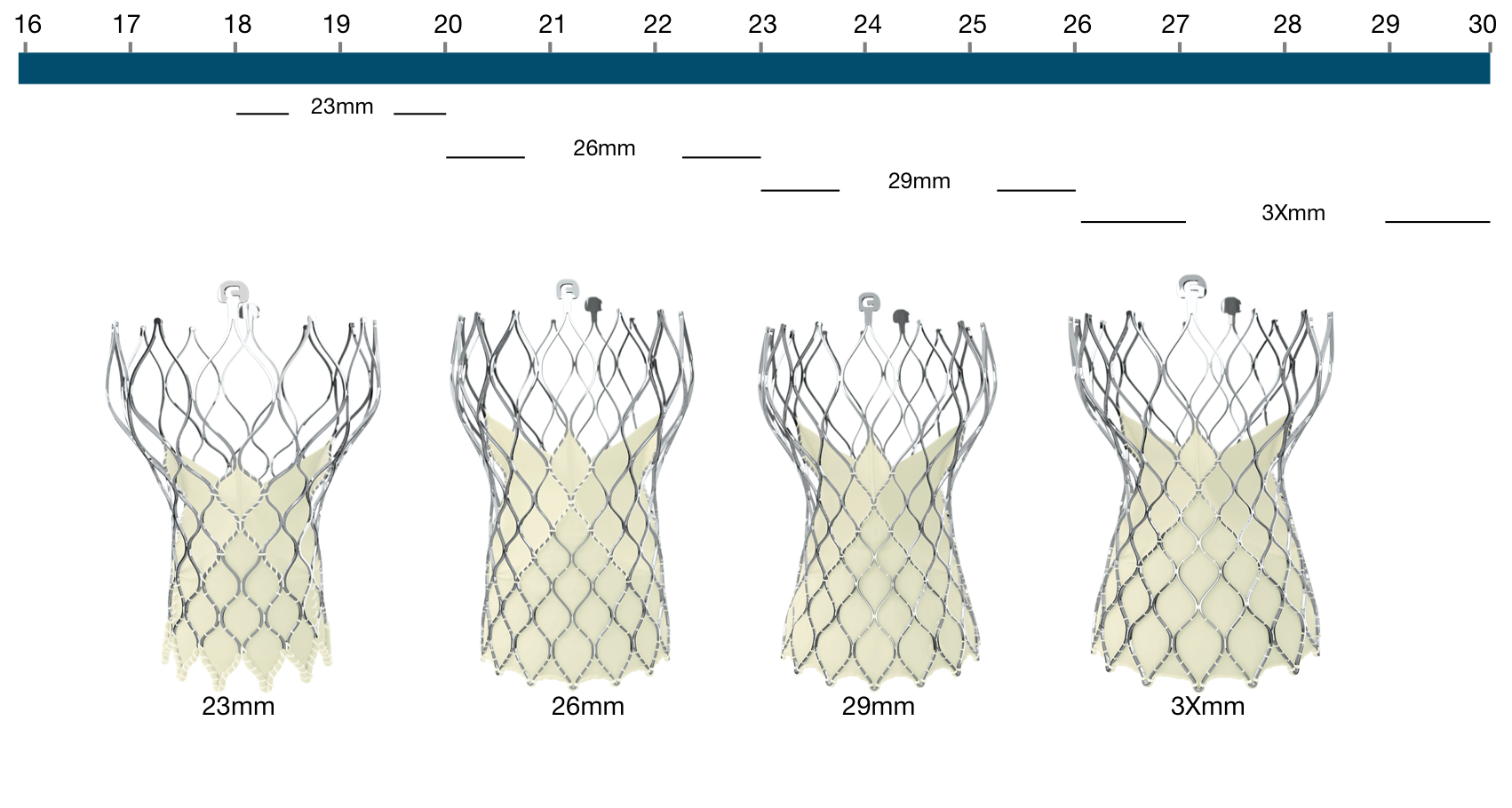
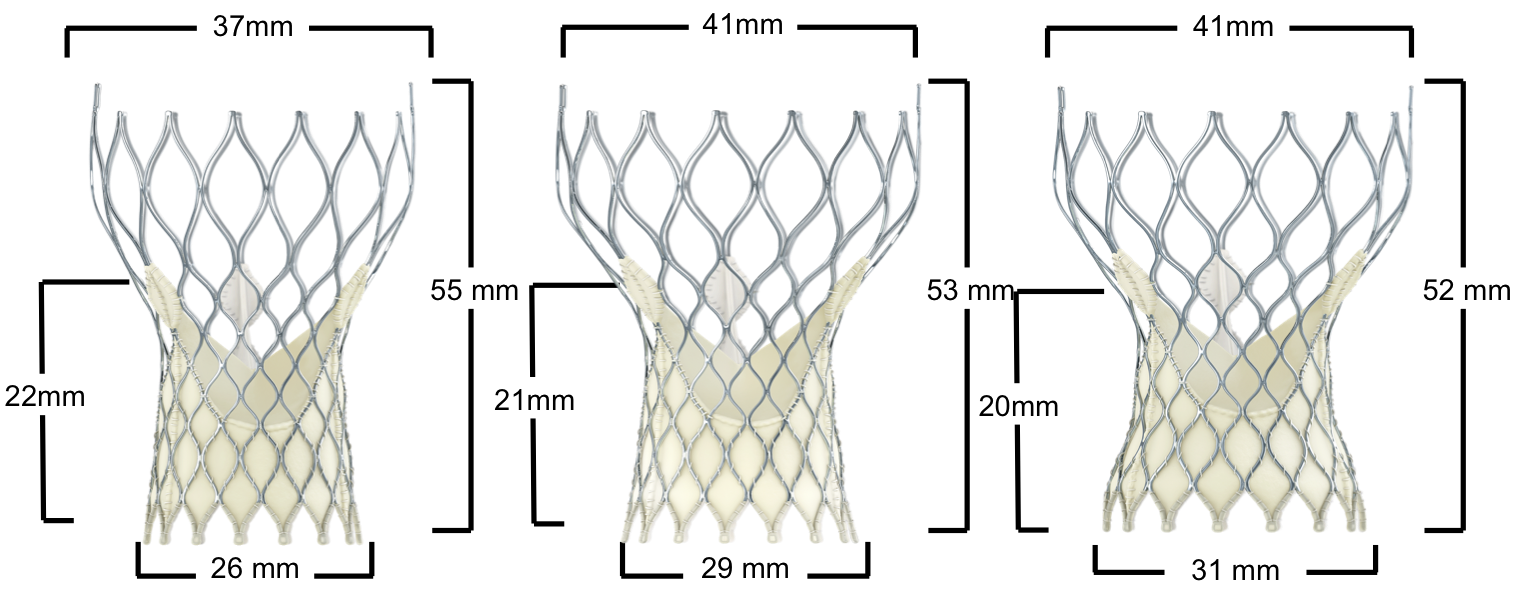
Figure 111. Medtronic CoreValve Evolut R transcatheter aortic valve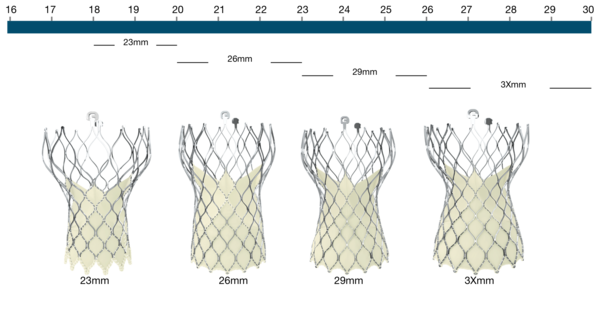
Medtronic CoreValve Evolut R | Native annulus diameter |
|---|---|
23mm | 18-20mm |
26mm* | 20-23mm |
29mm* | 23-26mm |
34mm* | 26-30mm |
General characteristics:
Design | Self-expanding |
Frame | Nitinol |
Leaflets | Porcine pericardium |
Valve size (mm) | 23 mm, 26 mm*, 29 mm*, 34 mm* |
Delivery system diameter - 1 (Figure 112) | 14 Fr equivalent |
Implantation access | Transfemoral, subclavian, direct aortic |
Repositionable | Yes |
Retrievable after being fully deployed | No |
Additional cuff to reduce AR | No |
Anchoring mechanism | No |
Tactile feedback during deployment | No |
Large stent cell design | Yes |
Markers to facilitate deployment | Yes |
Motorised delivery system | No |
Anticalcification technology | Yes |
CE mark status: Yes - 03/09/2014 for 23 mm
Click here for more information
CoreValve® Evolut™ Introduction
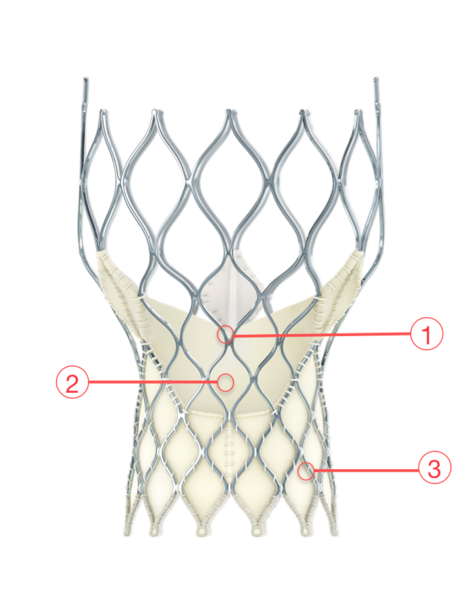
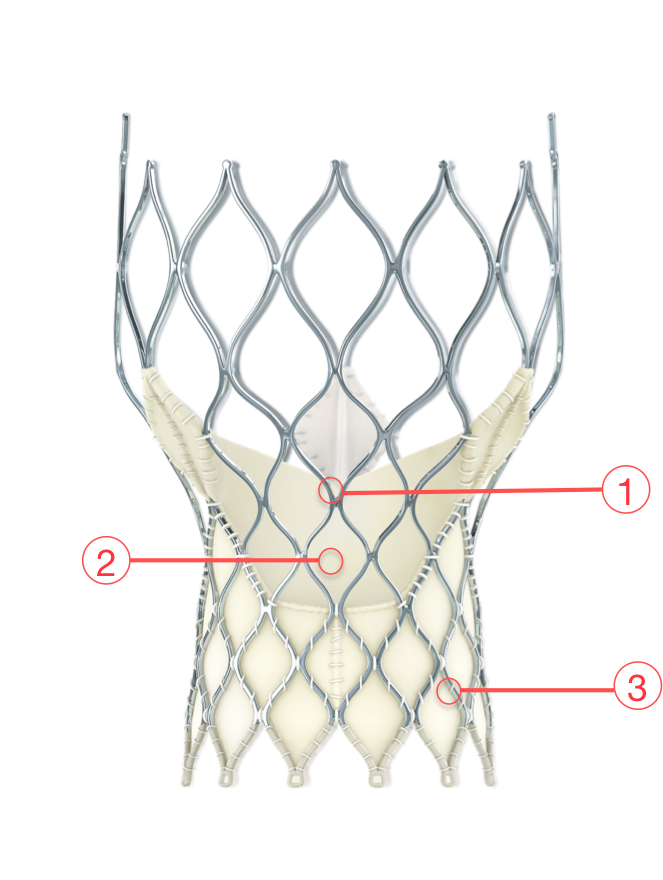
Figure 24. Medtronic CoreValve Evolut transcatheter aortic valve
1 - SUPRA-ANNULAR VALVE
Optimizes leaflet coaptation by preserving circularity at the level of valve function
2 - PORCINE PERICARDIAL TISSUE
Strong tissue, yet thin for low-profile delivery
3 - SELF-EXPANDING NITINOL FRAME
Conforms and seals at the non-circular annulus
Figure 25. Medtronic CoreValve Evolut transcatheter aortic valve dimensions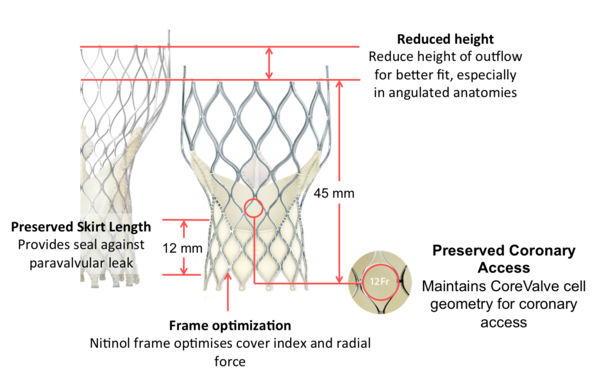
Medtronic CoreValve Evolut | Native annulus diameter |
|---|---|
23 mm | 18-20 mm |
General characteristic:
Design | Self-expanding |
Frame | Nitinol |
Leaflets | Porcine pericardium |
Valve size (mm) | 23 mm |
18 Fr | |
Implantation access | Transfemoral, subclavian, direct aortic |
Repositionable | Partially |
Retrievable after being fully deployed | No |
Additional cuff to reduce AR | No |
Anchoring mechanism | No |
Tactile feedback during deployment | No |
Large stent cell design | Yes |
Markers to facilitate deployment | Yes |
Motorised delivery system | No |
Anticalcification technology | Yes |
CE mark status: Yes - 26/09/2012
Special features:
- Customises anatomical fit via a tailored height and shape
- Conforms to the anatomy and promotes sealing with optimised cover index and radial force
- Preserves circularity and optimises leaflet coaptation at the level of valve function with supra-annular valve design
- Approved for use in degenerative surgical bioprostheses
Please Note: the CoreValve® Evolut™ and CoreValve® system are no longer available in Europe
CoreValve® Introduction
Over the last 10 years, the majority of surgically implanted aortic valves have been bioprosthetic. With a life expectancy of 10-20 years, the number of patients requiring redo surgery for failed bioprostheses is expected to increase. CoreValve and CoreValve Evolut provide options for heart teams who treat patients at high operative risk for redo surgery.
The Medtronic CoreValve™ system consists of 3 components: the transcatheter aortic valve (bioprosthesis), the delivery catheter system (catheter), and the compression loading system (CLS).
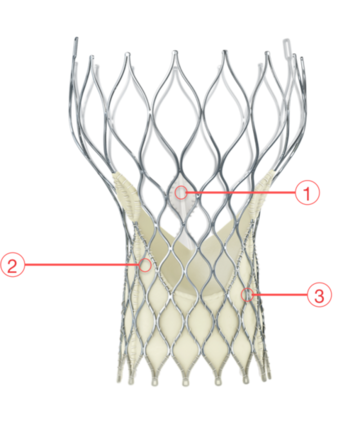
Figure 8. Medtronic CoreValve Transcatheter Aortic Valve
1 - SUPRA-ANNULAR VALVE
Optimizes leaflet coaptation by preserving circularity at the level of valve function
2 - PORCINE PERICARDIUM TISSUE
Strong tissue, yet thin for low-profile delivery
3 - SELF-EXPANDING NITINOL FRAME
Conforms and seals at the non-circular annulus
Figure 9. Medtronic CoreValve Transcatheter Aortic Valve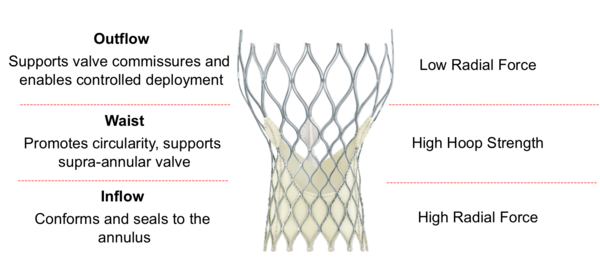
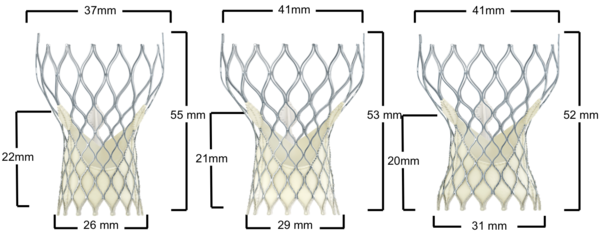
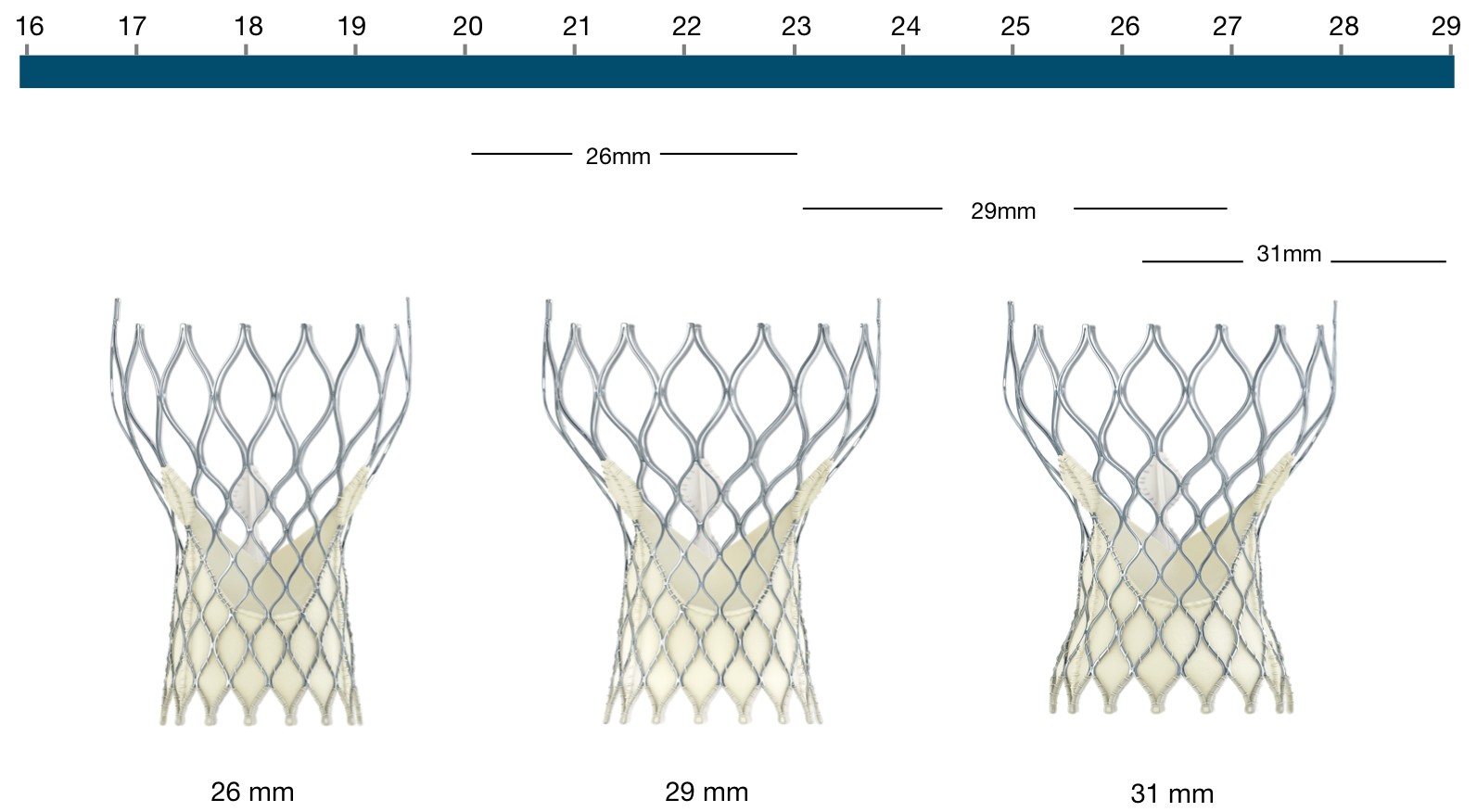
Figure 10. Medtronic CoreValve Transcatheter Aortic Valve Dimensions
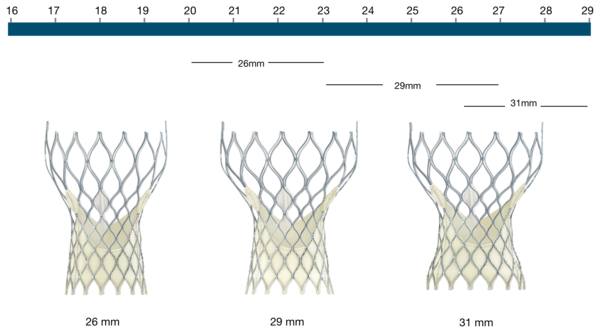
Figure 11. Medtronic CoreValve Transcatheter Aortic Valve native annulus diameter
CoreValve | Native annulus diameter |
|---|---|
26 mm | 20-23 mm |
29 mm | 23-27 mm |
31 mm | 26-29 mm |
General characteristics:
Design | Self-expanding |
Frame | Nitinol |
Leaflets | Porcine pericardium |
Valve size (mm) | 26 mm, 29 mm, 31 mm |
18 Fr | |
Implantation access | Transfemoral (Movie 2), subclavian, direct aortic |
Repositionable | Partially |
Retrievable after being fully deployed | No |
Additional cuff to reduce AR | No |
Anchoring mechanism | No |
Tactile feedback during deployment | No |
Large stent cell design | Yes |
Markers to facilitate deployment | Yes |
Motorised delivery system | No |
Anticalcification technology | Yes |
CE mark status: Yes - 16/05/2007
Special features:
- Preserves circularity and optimises leaflet coaptation at the level of valve function with supra-annular valve design
- Approved for use in degenerative surgical bioprostheses
CoreValve angiogram implantation:
- Medtronic CoreValve: Angiograms
- Medtronic CoreValve: Phantom model implantation
- Medtronic CoreValve: 3D rotational animation
CAUTION: Implantation of the Medtronic CoreValve™ system should be performed only by physicians who have received Medtronic CoreValve™ training.
The CoreValve® Evolut™ and CoreValve® system are no longer available in Europe