30 Oct 2017
Abbott Vascular: Portico™ Valve
Transcatheter aortic valve devices
The Portico™ Transcatheter Aortic Heart Valve offers outstanding control and accuracy for optimal patient outcomes with low rates of post-procedure pacemaker implantations, complications and paravalvular leak (PVL). The valve’s unique delivery system provides controlled deployment for greater accuracy and precise placement. The Portico™ transcatheter aortic heart valve is part of Abbott Vascular's transcatheter valve replacement portfolio.
Abbott Vascular has completed the acquisition of St. Jude Medical. Abbott vascular now sells the Portico™ valve.
Portico™ Valve introduction
The Portico™ transcatheter aortic heart valve is designed to be implanted in the native aortic heart valve without open-heart surgery and without concomitant surgical removal of the failed native valve. The valve is implanted using the Portico™ transfemoral delivery system.
Studies show that the Portico™ aortic valve has low rates of pacemaker implantation and complications and comparable PVL when compared to second-generation transcatheter aortic valve implantation (TAVI) valves.
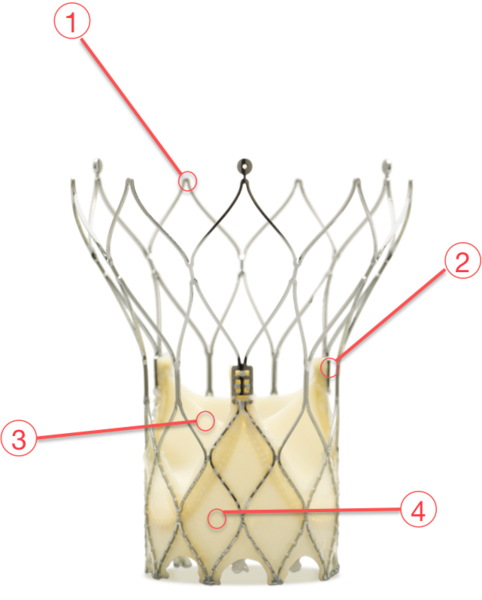
Figure 63. Portico aortic valve
1 - SELF-EXPANDING NITINOL FRAME
With a large cell design which allows access to coronaries and allows tissue to conform to the calcific nodules in the annulus to minimise PV leak
2 - NON-FLARED ANNULUS SECTION
Appropriate radial force and sealing
3 - BOVINE PERICARDIAL LEAFLETS
With Linx™ anticalcification treatment
4 - PORCINE PERICARDIAL SEALING CUFF
Conforms to native anatomy for sealing Linx™ anticalcification treatment
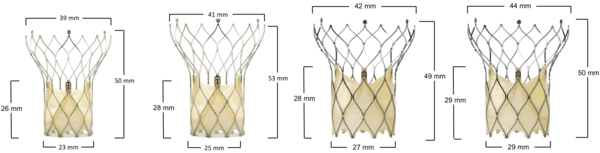
Figure 64. Portico aortic valve
Portico Valve | Valve area | Valve perimeter |
|---|---|---|
23 mm | 456 mm2 | 76 mm |
25 mm | 535 mm2 | 82 mm |
27 mm | 640 mm2 | 90 mm |
29 mm | 733 mm2 | 96 mm |
Portico 23 mm, 25 mm, 27 mm and 29 mm valves are ALL CE mark approved
Disclaimer: Photo not to scale
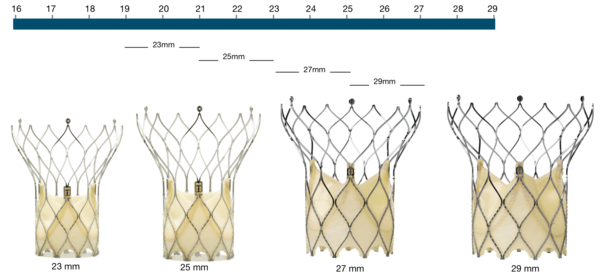
Figure 65. Portico aortic valve
Portico Valve | Native annulus diameter |
|---|---|
23 mm | 19-21 mm |
25 mm | 21-23 mm |
27 mm | 23-25 mm |
29 mm | 25-27 mm |
Native annulus perimeter* | Native annulus area* |
|---|---|
60-66 mm | 277-346 mm2 |
60-73 mm | 338-415 mm2 |
72-79 mm | 405-491 mm2 |
79-85 mm | 479-573 mm2 |
Portico™ Valve animation
General characteristics:
Design | Self-expanding |
Frame | Nitinol |
Leaflets | Bovine pericardium |
Valve size (mm) | 23 mm, 25 mm, 27 mm, 29 mm |
Delivery system diameter - 1 (Figure 66), | 18 Fr for 23 mm and 27mm (figure-3_70) |
Implantation access | Transfemoral (Movie 16), transapical, transaortic, subclavian |
Repositionable | Yes |
Retrievable after being fully deployed | No |
Additional cuff to reduce AR | Yes |
Anchoring mechanism | By design |
Tactile feedback during deployment | Yes |
Large stent cell design | Yes |
Markers to facilitate deployment | Yes |
Motorised delivery system | No |
Anticalcification technology | Yes |
CE mark status: 19/11/2012 (transfemoral)
Special features:
- Low valve height allowing for sealing without the valve extending deep into the LVOT to minimise conduction interference
- The large cells in the annulus section of the stent are designed to minimise the risk of PV leak
- Leaflet geometry designed to function optimally in both round (Movie 17) and elliptical configurations (Movie 26)
- Transfemoral delivery system and 23 mm and 25 mm valve CE approved. Transapical, Transaortic and Subclavian systems and 27 mm, 29 mm are investigational devices. Not available for sale in the U.S.A.


