30 results
EuroIntervention Achieves Record Impact Factor of 9.5
25 Jun 2025
Toulouse, FRANCE: Clarivate, publisher of the Journal Citation Reports (JCR) has announced the new Impact Factor for EuroIntervention of 9.5, putting it in the top percentile of academic publications published today.

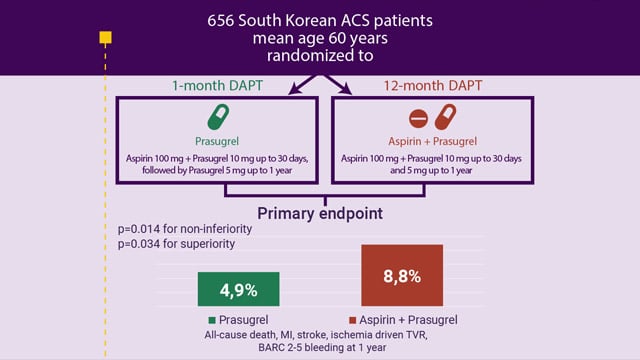
EuroPCR 2025 – One-month dual antiplatelet therapy followed by prasugrel monotherapy at a reduced dose: the 4D-ACS randomised trial
21 May 2025
Paris, France, 20-23 May 2025. The EuroPCR Course Directors have selected 3 major late-breaking trials that will be presented for the first time during the 2025 edition of EuroPCR. These trials were selected because of their design, results, and potential to impact practice, among them is the...
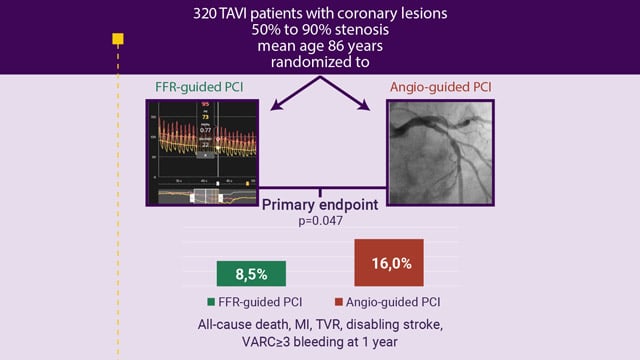
EuroPCR 2025 – The FAITAVI trial: angiography versus physiology-guided PCI in patients undergoing TAVI – 12-month follow-up data
21 May 2025
Paris, France, 20-23 May 2025. The EuroPCR Course Directors have selected 3 major late-breaking trials that will be presented for the first time during the 2025 edition of EuroPCR. These trials were selected because of their design, results, and potential to impact practice, among them is the...
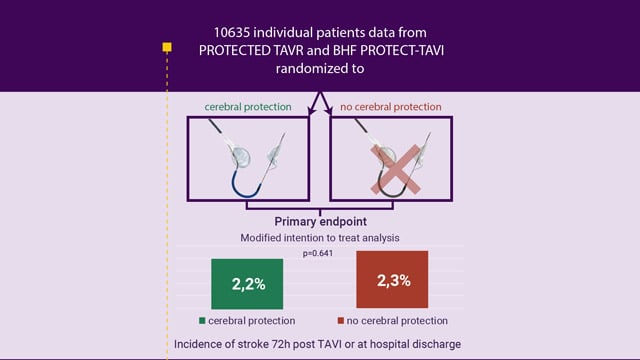
EuroPCR 2025 – Meta-Analysis of Individual Patient Data from the PROTECTED TAVR and BHF PROTECT-TAVI Trials
21 May 2025
Paris, France, 20-23 May 2025. The EuroPCR Course Directors have selected 3 major late-breaking trials that will be presented for the first time during the 2025 edition of EuroPCR. These trials were selected because of their design, results, and potential to impact practice, among which is the...
2025 Andreas Grüntzig Ethica Award presented to Lars Wallentin and Stefan James on behalf of Swedish cardiovascular registry experts
20 May 2025
The Andreas Grüntzig Ethica Award is traditionally given to those who have contributed in an extraordinary way to the PCR mission, that of serving the needs of patients by sharing knowledge, experience and practice in cardiovascular interventional medicine.

EuroPCR 2025 – The place to share solutions
07 May 2025
The annual World-Leading Course in interventional cardiovascular medicine is the official meeting of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Thousands of participants will once again be gathering to share their knowledge, skills and experience on 20-23 May 2025, at the Palais des Congrès in...

EuroPCR 2024 delivered an eye-opening focus on imaging guidance
24 May 2024
A record 12,100 participants from 130 countries have just taken part in the highly educational WorldLeading Course in interventional cardiovascular medicine. They enjoyed a unique opportunity to exchange their knowledge, skills and experience on a global scale.
Paris – France, 17 May 2024. As always, the thought-provoking...

EuroPCR 2024 – Andreas Grüntzig Ethica Award winners of EuroPCR 2024: Ottavio Alfieri and Frederick St Goar
15 May 2024
Paris, France, 15 May 2024. During each edition of EuroPCR, the Andreas Grüntzig Ethica Award, which recognises outstanding achievements and contributions to the cardiovascular field, is presented. On 15th May, at the Palais des Congrès, Porte Maillot in Paris, a special ceremony was held to bestow...

EuroPCR 2024 – One-month DAPT followed by 5-month Ticagrelor monotherapy in acute coronary syndromes with DCB - results from REC-CAGEFREE II
15 May 2024
Paris, France, 14-17 May 2024. The Course Directors have selected 3 major Late Breaking Trials (LBTs) that will be presented for the first time during the 2024 edition of EuroPCR. These trials were selected on account of their design, outcomes and potential to influence daily clinical...

EuroPCR 2024 – Short-term data from NOTION-2: TAVR versus SAVI for younger patients with aortic stenosis
15 May 2024
Paris, France, 14-17 May 2024. The Course Directors have selected 3 major Late Breaking Trials (LBTs) that were presented for the first time during the 2024 edition of EuroPCR. These trials were selected on account of their design, outcomes and potential to influence daily clinical practice....



