The PROTECTED TAVR study: Cerebral embolic protection during transcatheter aortic valve replacement
Reported from TCT 2022
Jonathan Curio provides his take on the PROTECTED TAVR study which was presented by Samir R. Kapadia at TCT 2022. The aim was to evaluate whether TAVR with cerebral embolic protection would reduce the rate of clinical stroke in transfemoral TAVR.
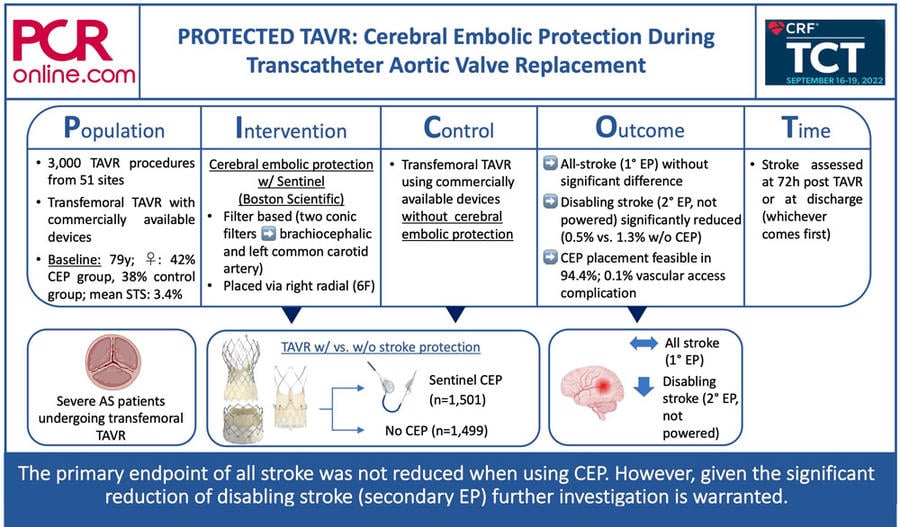
PICOT analysis of PROTECTED TAVR. Courtesy of Jonathan Curio, @CurioJonathan, Source: PCRonline.com
Why this study? – the rationale/objective
Transcatheter aortic valve replacement (TAVR) for patients with severe aortic stenosis has matured into a standardized procedure with high degrees of procedural success and safety. However, certain risks and adverse events remain, deserving specific attention, especially, as TAVR expands towards lower-risk often younger patients with longer life expectancies.
Here, stroke represents a potentially devastating complication with reported stroke rates of around 2-5%. These strokes mainly are procedural and are associated with significantly higher mortality as well as significantly higher needs for extended care and rehabilitation. Thus, cerebral embolic protection (CEP) devices have been developed, intended to protect the brain vasculature from debris evoked during delivery and device manipulation as well as actual valve implantation. As no larger randomized trial has evaluated the effect of CEP during TAVR, the PROTECTED TAVR study (NCT04149535) aimed to evaluate whether TAVR with CEP would reduce the rate of clinical stroke in transfemoral TAVR.
How was it executed? – the methodology
A total of 3,000 patients from 51 sites were 1:1 randomized to either TAVR with (n = 1,501) or without CEP (n = 1,499). All TAVR procedures were performed transfemorally with commercially available devices (64% Sapien 3 or iteration, 24% Evolut R/Pro (+), 7% ACURATE or iteration, 3% Portico or iteration, 1% Lotus Edge or iteration). For CEP the Sentinel Cerebral Protection System (Boston Scientific) was deployed.
The Sentinel device is a filter-based CEP solution, inserted via the right radial artery (6F profile). Two conic filters are sequentially deployed in the brachiocephalic artery and the left common carotid artery, catching debris during the procedure. After the procedure, the filters are recaptured, removing all collected material from the body. As a trial inclusion criterion and as per the device’s instruction for use patients had to have a brachiocephalic artery diameter of 9-15mm and a left common carotid artery diameter of 6.5-10mm.
- Neurological examinations were performed at baseline and 72h after the procedure or at discharge, if discharged earlier
- Primary endpoint was all stroke at 72h after the procedure or at discharge (whichever comes first)
- Prespecified additional endpoints were stroke severity (disabling vs. non-disabling), all-cause death, a composite of stroke + TIA + delirium, and vascular complications at CEP access site.
What is the main result?
Mean age of the trial population was 78.9 years, 42% of CEP patients were female, while only 38% of the control patients were female, and mean STS score was 3.4% (well-balanced with one third high, one third intermediate, and one third low operative risk).
- Device placement was feasible in 94.4% of patients (main reasons for failure were radial tortuosity or spasm or brachiocephalic/subclavian tortuosity)
- The primary endpoint of all stroke at 72h/discharge was without significant difference in the two groups (2.3% in CEP vs. 2.9% in control group, p=0.30)
- Analysis of the prespecified additional endpoints revealed a significant reduction of disabling stroke by CEP (0.5% vs. 1.3% in control group), driven by a lower incidence of ischemic strokes with the use of CEP
- CEP access site related vascular complications occurred in 0.1%
- All-cause mortality at 72h/discharge was without differences between the groups (0.5% in CEP vs. 0.3% in control group)
Critical reading and the relevance for clinical practice
The eagerly awaited PROTECTED TAVR trial will undoubtedly have an important impact on the field of CEP. Despite the concept’s intriguing logic, previous studies failed to show a meaningful benefit when adding CEP to TAVR. Also in this large prospective randomized trial evaluating clinical stroke 72h after TAVR or at discharge (if earlier than 72h) no difference between CEP and TAVR without CEP regarding all stroke could be identified.
As a secondary endpoint a significant reduction of disabling stroke when using CEP was reported (number needed to treat: 125), driven by a reduction of, especially, ischemic events, suggesting this being a direct result of the CEP system’s mode of action. Disabling stroke represents a devastating outcome feared by physicians, patients, and relatives alike and, thus, these results understandably may lead to the well-meant intention to use CEP across the broad range of patients treated in the trial. However, it is important to note that the trial was not powered for this endpoint.
The population treated seems well representative of a broad range of current TAVR practice. Of note, a higher number of females, a known risk factor for stroke, was enrolled in the CEP arm. Analyses of different sub-groups (including bicuspid vs. tricuspid valves, valve calcification, pre-dilatation, prior cerebrovascular events) could not identify any patient or procedure characteristics that might favor the use of CEP to reduce overall stroke.
Importantly, the use of CEP appears to be very safe and does not add any risk or harm to the TAVR procedure. Device placement was feasible in 94.4% of the enrolled patients, however, the rate of patients excluded during screening based on anatomy unfavorable for device placement was not reported.
The studied filter-based Sentinel device represents a unique CEP concept. Other devices covering all three branches of the aortic arch (then not collecting the debris, but deflecting it to the downstream circulation) or large aortic filters capturing not only debris directed to the cerebral vasculature but the whole body, are under development or clinical testing and may result in different outcomes.
Given the great relevance of disabling stroke, the findings of PROTECTED TAVR warrant further evaluation. In fact, the BHF PROTECT-TAVI trial (ISRCTN16665769) is ongoing and enrolling an estimated number of 7,730 patients. As the trial has the same primary outcome (stroke at 72h/discharge) an individual patient data meta-analysis is already planned and will bring further, hopefully final, answers regarding the benefits of CEP.
Until then, taken together, with the findings of PROTECTED TAVR the faith of CEP still remains a case that cannot be successfully closed, nor it is a story ended or an approach to abandon – rather this trial is a further chapter added to a story that needs more exploration given the key importance that stroke, and especially disabling stroke, has for patients.







No comments yet!