Percutaneous transseptal transcatheter mitral valve replacement using a dedicated balloon-expandable valve: 1-year outcomes from the ENCIRCLE trial using the SAPIEN M3
Reported from TCT 2025
Jonathan Curio provides his take on the ENCIRCLE Trial presented by David Daniels at TCT 2025 in San Francisco.
Designed by: Jonathan Curio
Source: PCRonline.com
Why this study? - the rationale/objective
Despite the continued success of mitral transcatheter edge-to-edge repair (M-TEER) for the treatment of symptomatic mitral regurgitation (MR) in patients at increased surgical risk, some limitations of this interventional approach, such as anatomical M-TEER ineligibility, as well as residual or recurrent MR still remain1.
Transcatheter mitral valve replacement (TMVR) bears the promise to potentially overcome several of these limitations as it could be used in anatomies challenging for M-TEER (such as heavy calcification, multiple MR jets or a small mitral valve area) and likely will more or less abolish MR. However, albeit the first-in-human TMVR procedure has been performed as early as 2012, until today, broad application is lacking. This is due to a high screen-failure rate (often caused by potential risk of LVOT-obstruction) and a high procedural risk given the fact that the (until recently) only CE-marked device had to be implanted via a transapical approach2,3.
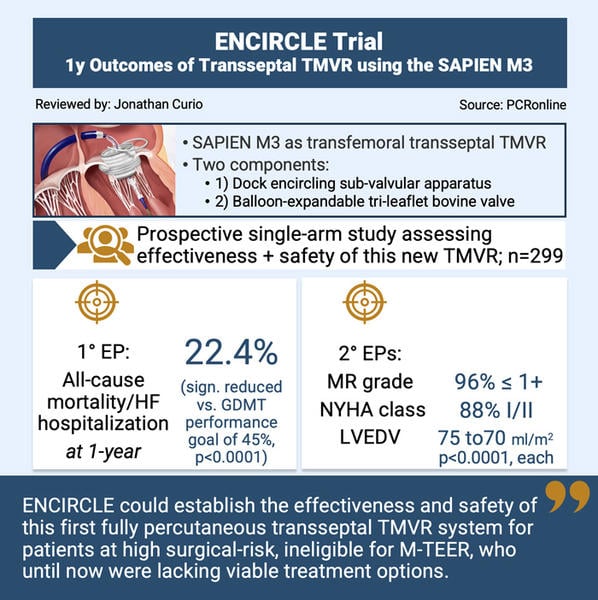
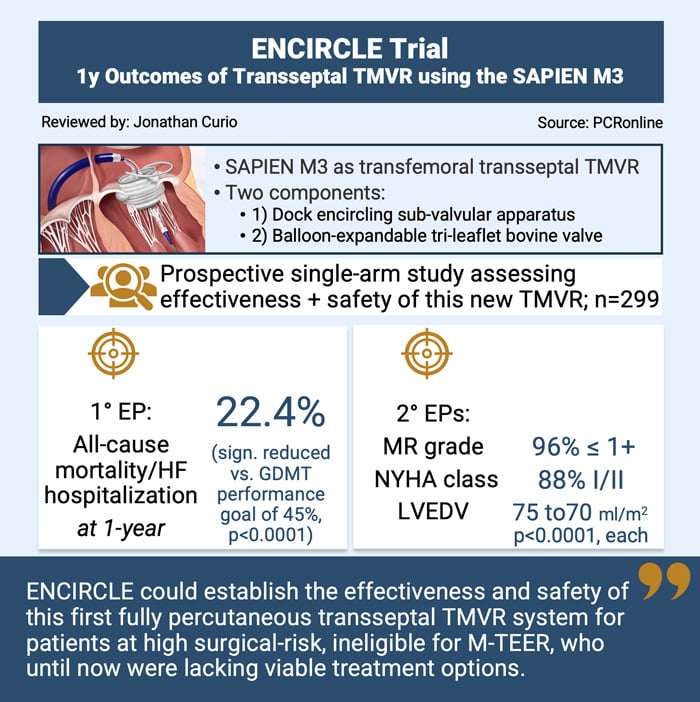
Against this background, the SAPIEN M3 system as a novel TMVR system allows for transseptal delivery of a two-component balloon-expandable valve. In a first procedural step, a dock, encircling the sub-valvular apparatus, further stabilised via an atrial arch, is implanted. As a second step, a tri-leaflet bovine pericardial balloon-expandable valve, an adopted version of the established aortic SAPIEN platform, is placed within this docking system.
The ENCIRCLE trial is a pivotal prospective single-arm, multicenter study evaluating the effectiveness and safety of the SAPIEN M3 system.
How was it executed? – the methodology
The ENCIRCLE trial was a single-arm prospective study enrolling patients with symptomatic MR ≥ 3+, who were unsuitable for open heart surgery or M-TEER, to be treated with TMVR using the SAPIEN M3. The trial included 299 patients, excluding patients with anatomy unsuitable for SAPIEN M3, LVEF < 25 %, severe right ventricular dysfunction, severe pulmonary hypertension or renal insufficiency.
Patients were followed at 30 days, 6 months, 1 year, and will be follow-up annually through 5 years.
Endpoints of the trial included:
- Primary efficacy endpoint: 1-year composite of all-cause death or heart failure hospitalisation (tested against a pre-specified performance goal of 45 % at 1 year, based on optimised medical therapy arms of M-TEER trials)
- Secondary endpoints: 1-year improvements in NYHA class, MR grade and LVEDVindex
- Additionally clinical outcomes such as stroke, major bleeding, device thrombosis, and hemolysis requiring intervention were assessed at 30 days and 1 year
What is the main result?
Mean age of the trial population was 75.5 years and 49.2 % of the patients were female. The STS risk-score for operative mortality was 6.6 %. Congestive heart failure was present in 75 % of patients and 71 % presented in NYHA class III/IV. 35 % of patients suffered from primary MR and 58 % from secondary MR (the vast majority with a ventricular pathomechanism).
- According to the primary endpoint, TMVR using the SAPIEN M3 significantly outperformed the performance goal, showing a rate of all-cause mortality or heart failure hospitalisation of 25.2 % at 1-year (p < 0.0001); all-cause death was 13.9 % and heart failure hospitalisation rate 16.7 %
- Secondary endpoints at 1-year showed: significant improvement in MR grade (96 % MR ≤ 1+, p < 0.0001), improvement in NYHA class (88 % NYHA class I/II, p < 0.0001), and positive remodeling of the left ventricle (LVEDVi reduction from 75.1 to 70.8 ml/m2, p < 0.0001)
- At 30 days and 1 year, disabling stroke was present in 1.7 % and 3.9 %, major bleeding in 8.7 % and 18.5 %, device thrombosis occurred in 2.3 % and 6.7 %, and hemolysis requiring intervention was seen in 4.3 % and 7.1 %, respectively
Critical reading and the relevance for clinical practice
The results of the ENCIRCLE trial mark a milestone in the over one-decade long story of catheter-based valve replacement to treat mitral regurgitation, for the first time establishing the lasting procedural effectiveness and safety of a transseptal system, namely the SAPIEN M3.
After the tremendous success story of transcatheter valve replacement in the aortic space, until now, results on the mitral valve have been lagging behind. This was likely driven by the far more complex mitral valve apparatus - comprising not only the valve leaflets itself, but, at least as important, the continuum of left atrium and left ventricle for anchoring of the device, as well as the proximity to the left ventricular outflow tract, at risk of obstruction after TMVR. Only a single transapical system, thus far, could find its way into clinical practice. The SAPIEN M3 platform now seems to solve several of these issues. First, it is a fully percutaneous transseptal device. Second, the two-component platform with its dock encircling the sub-valvular apparatus (potentially, helping to clear the neo-LVO), further stabilised by an atrial arch, provides a stable landing zone for a straight-forward balloon-expandable delivery.
While it might be reasonable to assume that such transseptal system will have natural advantages over a more invasive transapical TMVR approach, its role in the armamentarium to treat MR, especially in comparison to M-TEER, remains to be studied and identified. The screen failure rate of 72 % (also with this device design still 35 % excluded for solely anatomic reasons) in a population that is not eligible for surgery or M-TEER remains high and leaves room for discussion if this therapy can be applied in the broader population of MR patients. While the clinical results of the patients treated with TMVR in the ENCIRCLE trial may compare favorably with results seen in landmark M-TEER trials, continued device iterations and tremendous learnings regarding procedural characteristics have further improved M-TEER outcomes, often guaranteeing post-procedural MR ≤ 1+ as well. Thus, future potential studies comparing TMVR using this device with M-TEER as well as with optimised guideline directed medical therapy would be of high interest. Here, of note, other than M-TEER, TMVR is mostly agnostic to the mechanism of MR, able to treat primary as well as secondary MR in an equal manner.
In the meanwhile, it will be important to better understand the longer-term performance of this novel TMVR system. As of now, thrombosis rate at 1-year ranges at 6.7 %, while it is known that the mitral valve space carries a higher thrombotic risk than the aortic. This may influence not only the performance, but also the durability of this platform, which remains to be studied in the longer-term follow-up of the trial.
In conclusion, the ENCIRCLE trial could establish the safety and effectiveness of the first percutaneous transseptal TMVR system, paving the way for a new chapter in the treatment of MR patients, some of whom until now still had no other viable treatment option. Future studies will elucidate its role in the growing armamentarium to treat clinically relevant MR.
References
- Lim DS, Herrmann H, Grayburn P, Koulogiannis K, Ailawadi G, Williams M et al. Consensus Document on Non-Suitability for Transcatheter Mitral Valve Repair by Edge-to-Edge Therapy. Structural Heart 2021;5:227-233.
- Coisne A, Pontana F, Tchétché D, Richardson M, Longère B, Vahdat O et al. Transcatheter mitral valve replacement: factors associated with screening success and failure. EuroIntervention 2019;15:e983-e989.
- Wild MG, Kreidel F, Hell MM, Praz F, Mach M, Adam M et al. Transapical mitral valve implantation for treatment of symptomatic mitral valve disease: a real-world multicentre experience. Eur J Heart Fail 2022;24:899-907.




No comments yet!