GLORIOUS - Efficacy of restrictive versus liberal oxygenation or Exenatide in patients undergoing coronary artery bypass grafting or aortic valve replacement – a randomised clinical trial
Reported from AHA 2024
Edoardo Zancanaro provides his take on the GLORIOUS trial presented by Sebastian Wiberg at AHA 2024 in Chicago.
Why this study – The rationale/objective
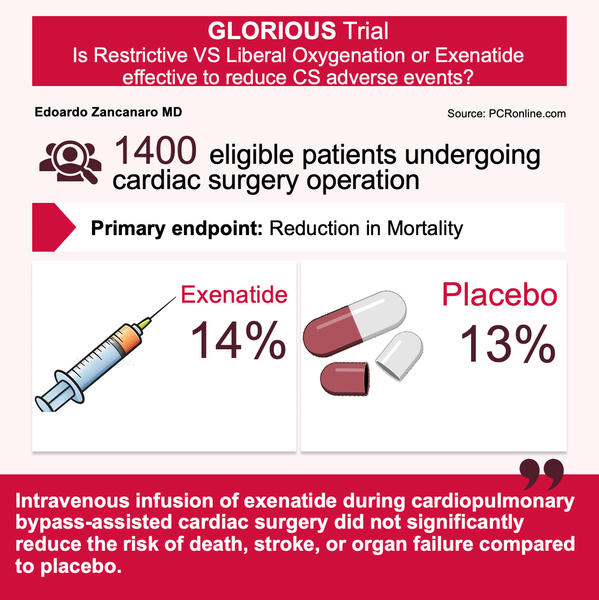
The GLORIOUS trial was conducted to address an important question in the management of patients undergoing cardiac surgery operation, with key attention to aortic valve replacement or coronary artery bypass grafting.
In particular, they used Exenatide that was first approved in April 2005 by the US Food and Drug Administration (FDA) as an adjunctive therapy to improve blood sugar control in patients with type 2 diabetes (T2D). On the other side, they used different oxygenation therapy (liberal vs restrictive) to prove the possible outcomes.
How was it executed – The methodology
The GLORIOUS trial was a randomised, double-blind, placebo-controlled clinical trial that enrolled approximately 1,400 adults at a heart center in Denmark between 2016 and 2021. This population was monitored during surgery—investigators collected nearly 6 years of follow-up data for post-surgical outcomes, including death, kidney failure requiring renal replacement therapy, stroke, new-onset heart failure, and hospital readmission for heart failure or other cardiovascular causes.
Study Protocol:
The trial employed a 2 x 2 factorial design to evaluate:
- Intravenous Exenatide infusion:
- Objective: to assess whether Exenatide, a glucagon-like peptide-1 (GLP-1) receptor agonist, could reduce complications such as death, stroke, or organ failure during and after cardiac surgery.
- Method: participants were randomised to receive either a 6-hour and 15-minute infusion of Exenatide (17.4 μg) or a placebo initiated at the time of anesthesia prior to surgery.
- Oxygenation strategies:
- Objective: to determine if a restrictive oxygenation strategy during cardiopulmonary bypass could reduce mortality or morbidity from renal failure, stroke, or heart failure.
- Method: participants were assigned to either a restrictive oxygenation group (administered oxygen fraction of 50 %) or a liberal oxygenation group (administered oxygen fraction of 100%) during cardiopulmonary bypass and the first hour of weaning off bypass.
What are the Main Results?
1. Exenatide Infusion:
The study found no significant differences in outcomes between the Exenatide and placebo groups over an average follow-up period of nearly six years.
- Mortality: 14 % in the Exenatide group vs. 13 % in the placebo group.
- Stroke: 5.8 % in the Exenatide group vs. 4.8 % in the placebo group.
- New or worsening heart failure: 9.8 % in the Exenatide group vs. 10 % in the placebo group.
- Acute kidney injury: 4.8 % in the Exenatide group vs. 5.3 % in the placebo group.
2. Oxygenation Strategies:
Neither the restrictive nor liberal oxygenation strategies resulted in significant differences in the primary endpoint of mortality, stroke, renal failure requiring replacement, or new-onset/worsening heart failure. Event curves for both groups were virtually superimposable, indicating no benefit from either strategy.
The GLORIOUS trial concluded that:
- Intravenous infusion of Exenatide during cardiopulmonary bypass-assisted cardiac surgery did not significantly reduce the risk of death, stroke, or organ failure compared to placebo.
- A restrictive oxygenation strategy during cardiopulmonary bypass did not improve clinical outcomes compared to a liberal oxygenation approach.
These findings suggest that neither intervention offers significant benefits in the context studied. However, the researchers noted the need for further studies to explore different GLP-1 analogs, longer administration periods, or larger doses, as well as to identify optimal oxygenation strategies during cardiac surgery.
Critical Reading and the Relevance for Clinical Practice
The GLORIOUS trial was conducted to assess a very important topic at the moment which is the try to reduce the effect of cardiac surgery over patients treated.
The trial, articulated into two different option for patients, did not find significant results in use of GLP-1 analogs or in different oxygenation strategy.
This brings some important consideration but for sure the most relevant is that peri-operative management for cardiac surgery is still a vast field that needs to be addressed.
Lately, Landoni et all1 addressed a specific question, also part of the GLORIOUS trial, on the fact that acute kidney injury (AKI) is a serious and common complication of cardiac surgery, for which reduced kidney perfusion is a key contributing factor. They perform a randomised control trial in which they infused intravenous amino acids to increase kidney perfusion and recruit renal functional reserve. They discovered that among adult patients undergoing cardiac surgery, infusion of amino acids reduced the occurrence of AKI.
Of course, the two trial cannot be compared but in GLORIOUS, AKI was seen in 4.8 % in the Exenatide group vs. 5.3 % in the placebo group while in PROTECTION, AKI occurred in 474 patients (26.9 %) in the amino acid group and in 555 (31.7 %) in the placebo group (relative risk, 0.85; 95 % confidence interval [CI], 0.77 to 0.94; P = 0.002).
Also, listening to the main author words during the commentary part of the presentation (Sebastian Wiberg, MD, PhD), even though, these two current strategies presented and tested in the aforementioned trial don’t bring difference in outcomes of cardiac surgery patients, this still make a path to understand on what can be useful and what not.
In conclusion, the GLORIOUS trial didn’t bring significant evidence of possible positive outcomes for the treatment studied, but make a clearer explanation of what might not be beneficial for these type of cohort patients.
Reference:
- Landoni G, Monaco F, Zangrillo A, Bellomo R; PROTECTION Study Group. A Randomized Trial of Intravenous Amino Acids for Kidney Protection. N Engl J Med. 2024 Aug 22;391(8):687-698. doi: 10.1056/NEJMoa2403769. Epub 2024 Jun 12. PMID: 38865168.






No comments yet!