Percutaneous coronary intervention of native coronary artery versus saphenous vein graft in patients with prior coronary artery bypass graft surgery (PROCTOR)
Reported from TCT 2025
Kalaivani Mahadevan and Trisha Singh provide their take on the PROCTOR study presented by Ruben de Winter at TCT 2025, in San Francisco.
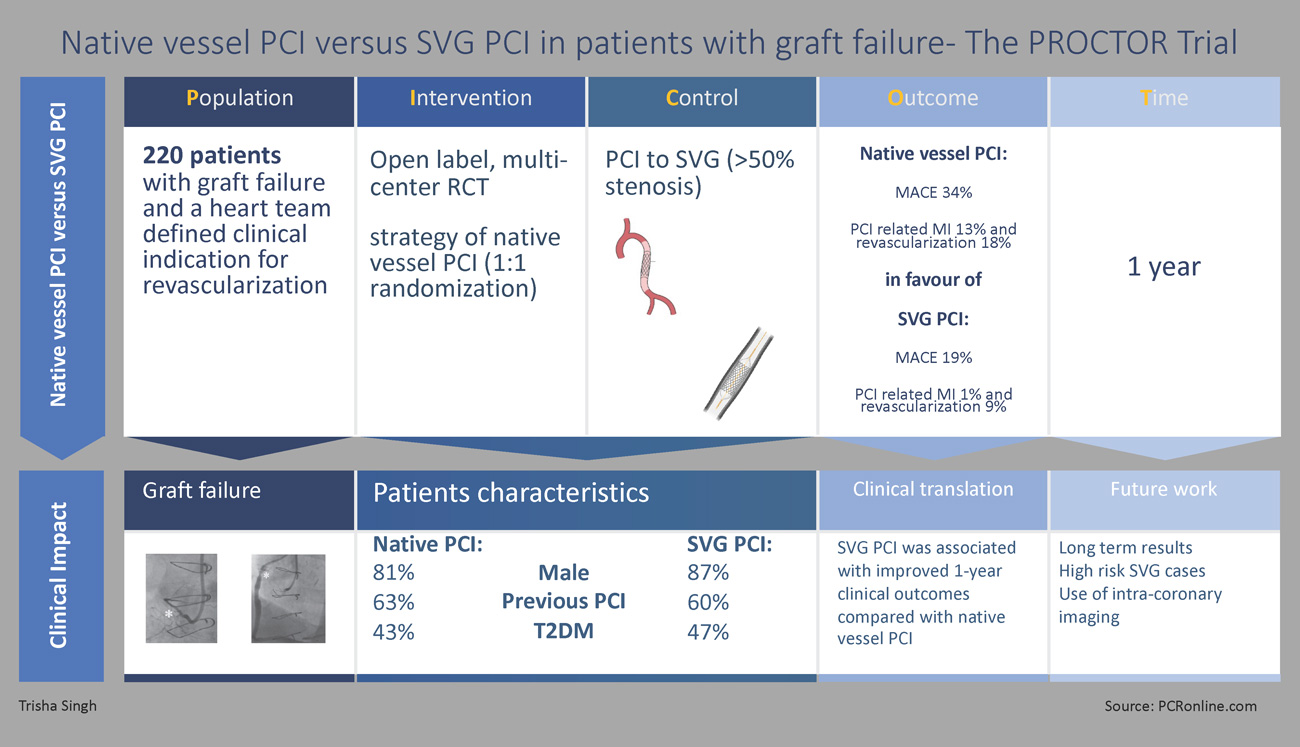
Designed by Trisha Singh.
Source: PCRonline.com
Study rationale and background
Coronary artery bypass grafting (CABG) remains an important revascularisation strategy for multivessel coronary artery disease and other complex coronary pathologies1. However, saphenous vein grafts (SVGs) exhibit accelerated progression of atherosclerosis both in the conduit and the native vessel, limiting durability and posing distinct challenges to graft failure management2.
Despite advances in surgical techniques and pharmacotherapy, SVG failure frequency is up to 50 % at 10 years and is associated with adverse outcomes3,4. Treatment options, dependent on anatomy, include percutaneous coronary intervention [PCI] of the graft or of the native coronary which commonly involves one or more chronic total occlusions [CTO].
SVG PCI presents unique challenges. The conduit is subject to adaptive intimal hyperplasia and accelerated atherosclerosis, and often demonstrates lesion friability, vessel ectasia and high thrombus burden (Figure 1). These increase both the risk of distal embolisation, no-reflow, and periprocedural MI, as well as downstream SVG in-stent restenosis [ISR] and target lesion [TLR] and vessel revascularisation [TVR]2,3. However, post-CABG native vessel PCI is complex, with long lesion length, extensive calcification, negative vessel remodelling and higher prevalence of CTOs5.
Observational studies have shown better short- and long-term outcomes with native vessel PCI, based on which current expert consensus reviews and guidelines advise native vessel PCI with a 2aB recommendation3,6. To address uncertainty around best practice, The PROCTOR trial was designed to test the hypothesis that a strategy of native vessel PCI is superior to SVG PCI for the first time, in a randomised setting7.
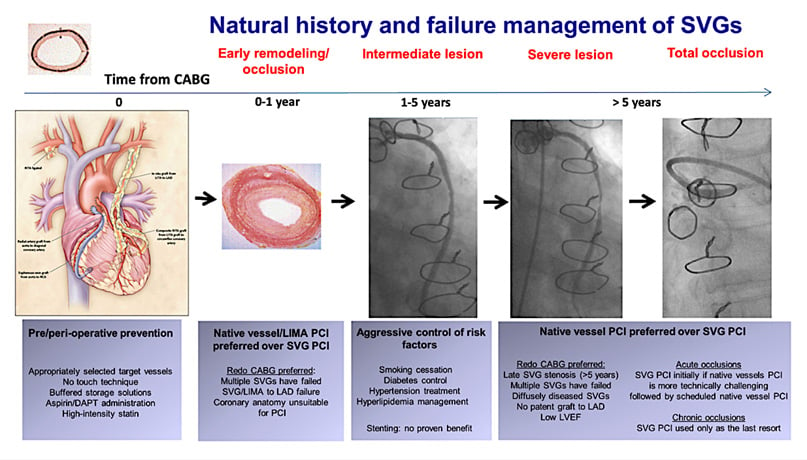
Figure 1: Natural history and failure management of saphenous vein grafts
Figure courtesy of Xenogiannis et al. Circulation. 2021, 144, 728-745
Study design
Design
Prospective, multicenter, randomised controlled trial [RCT].
Population
Patients presenting with SVG failure (> 50 % stenosis) and a clinic indication for revascularisation deemed suitable for both SVG and native vessel PCI at HEART MDT.
Intervention groups
Native vessel PCI and SVG PCI.
Endpoints
For the 1–year primary endpoint, a composite MACE was utilised (consisting of all-cause mortality, non-fatal target territory MI, and clinically driven target territory revascularisation).
Secondary endpoints were individual components of the MACE, target vessel failure [TVF], target lesion revascularisation [TLR], renal failure requiring renal replacement therapy and patient-reported quality of life (CCS/RDS).
Follow-up duration
5 years with planned interim analyses at 3 years.
Statistical analyses:
Kaplan-Meier curves with log-rank testing utilised to compare event-free survival for MACE and it’s individual components alongside Cox proportional hasard regression analyses to calculate hasard ratios, between the two treatment groups.
Results
Key patient, lesion and procedural demographics are summarised in Figure 2. Baseline characteristics for cardiovascular risk factors, cardiac co-morbidities and prognostic pharmacotherapies were well matched across cohorts.
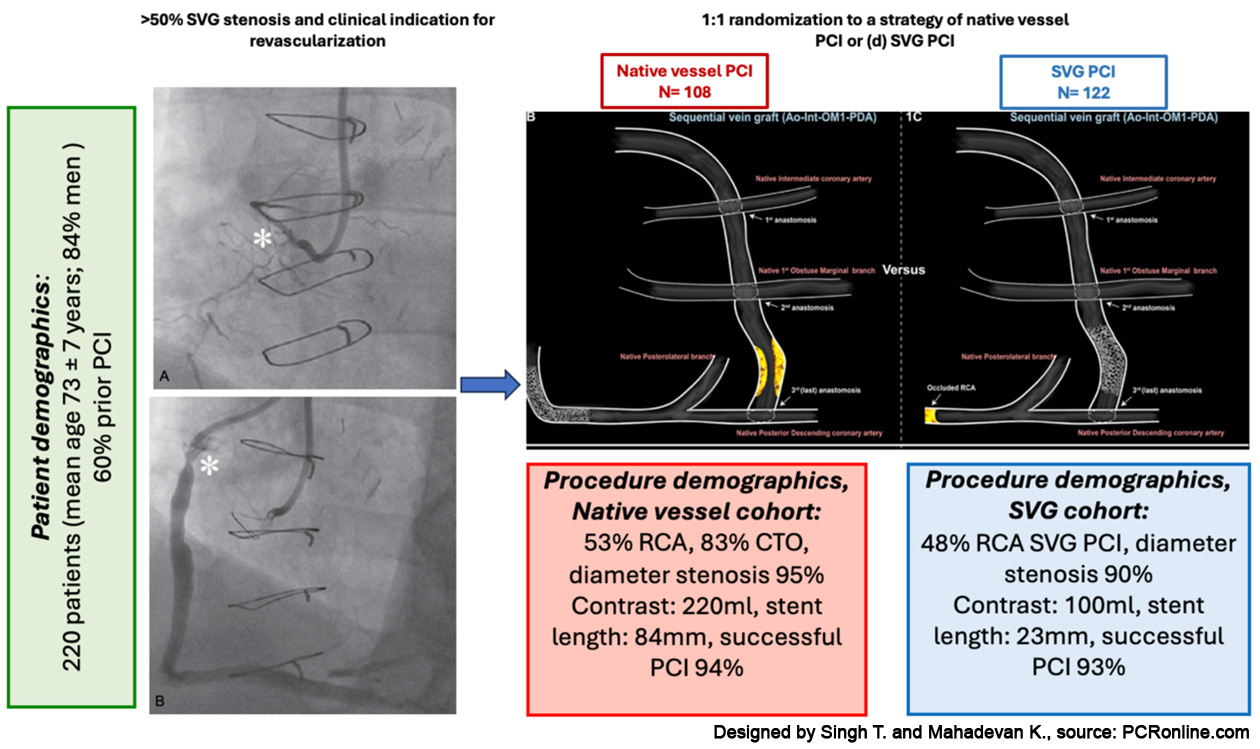
Figure 2: Patient, lesion and procedural demographics
Designed by Singh T. and Mahadevan K., source: PCRonline.com
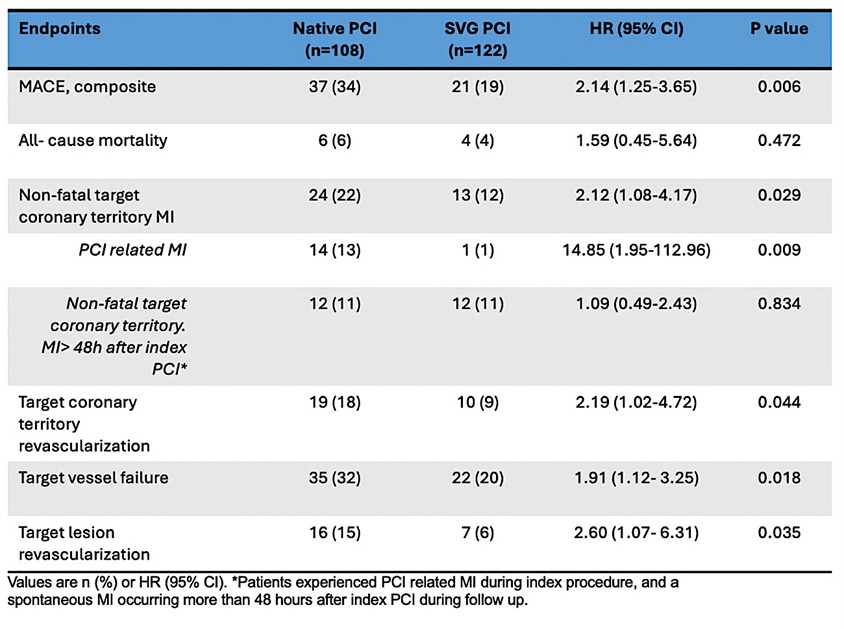
At one-year, the primary endpoint of composite MACE occurred in 37 patients (34 %) in the native vessel PCI group vs 21 patients (19 %) in the SVG PCI group (HR: 2.14; 95 % CI: 1.25-3.65; P = 0.006) (Figure 3)8. Whilst there was no significant difference in all-cause mortality, both non-fatal target coronary territory MI (HR: 2.12; 95 % CI: 1.08-4.17; P = 0.029) and clinically driven target coronary territory revascularisation (HR: 2.19; 95 % CI: 1.02-4.72; P = 0.044) occurred more frequently in the native vessel PCI cohort (Figure 3). Furthermore, the incidence of TVF and TLR was also higher in the native PCI cohort (p = 0.018 and 0.035 respectively) (Table 1)8.
SVG PCI was associated with better 1-year clinical outcomes compared with native vessel PCI, primarily driven by lower rates of PCI-related MI and clinically driven target coronary territory revascularisation.
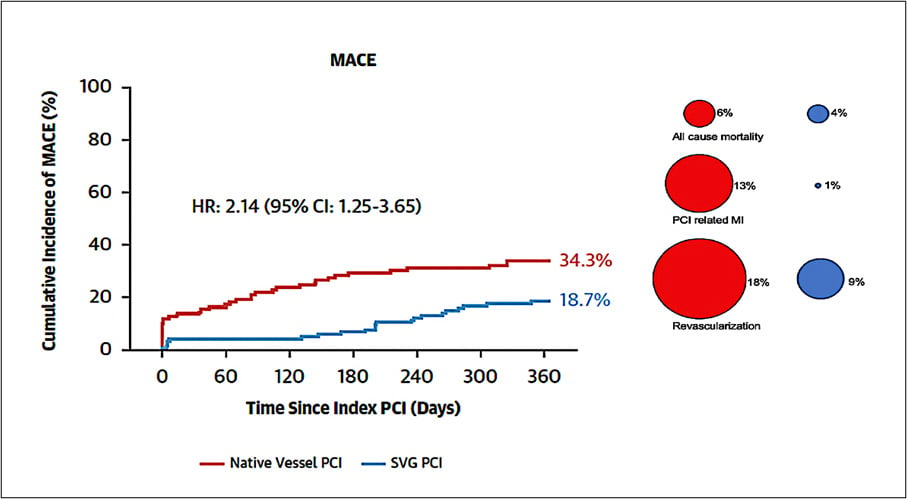
Figure 3: Kaplan-Meier curve for the rate of the composite endpoint of all-cause mortality, nonfatal target coronary territory MI, or clinically driven target coronary territory revascularisation at 1 year
Figure courtesy of De Winter et al. J Am Coll Cardiol 2025 Oct 28:S0735-1097(25)09477-X
Table 1: Intention to treat endpoint results
Discussion and critical analysis
The PROCTOR trial was designed with the intent to investigate and understand the longer-term durability of differing revascularisation strategies in post–CABG patients. This was based on well understood challenges surrounding recurrent SVG failures and the hypothesis that native vessel revascularisation had the potential for a superior and more durable outcome.
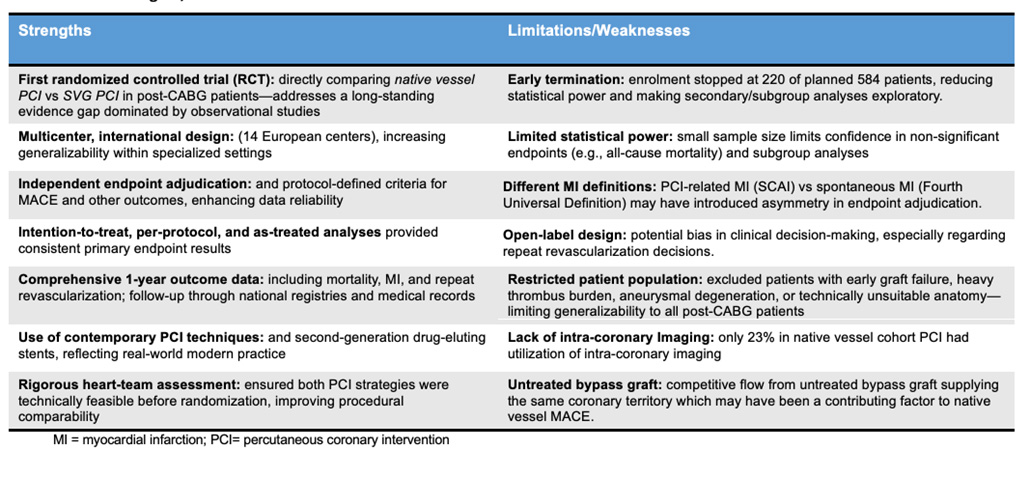
Table 2 summarises study strengths, limitations and weaknesses. This was the first RCT addressing the uncertainty around optimal revascularisation (native vessel versus SVG PCI) in post–CABG patients. Numerous centres were included, with independent endpoint adjudication increasing outcome validity, and comprehensive data analysis. Of note, the study was terminated early due to funding restraints and slow recruitment, reducing statistical power and rendering secondary endpoints predominantly exploratory. Further the open-label design of the study could have led to potential bias in clinical decision-making including for repeat revascularisation.
Table 2: Study strengths, limitations and weaknesses
Native vessel PCI post CABG currently carries a 2aB guidance based on historic observational data demonstrating favourable outcomes over SVG PCI9-10. We explore potential explanations for the opposite and perhaps unexpected result observed in PROCTOR.
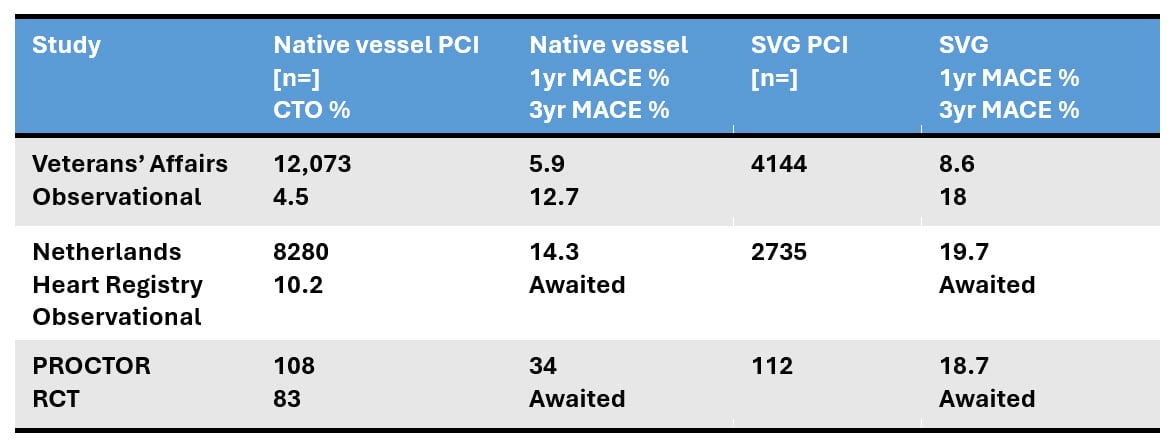
First, the native vessel PCI cohort contained disproportionately high rates (83 %) of CTOs as the PCI target lesion versus prior registries (in which selection bias against treatment of native vessel CTO lesions in favour of graft PCI or conservative therapy, is possible) (Table 3). Toma et al. have reported the association of increased peri-procedural myocardial injury with CTO PCI, a phenomenon found to be incrementally higher with retrograde crossing/completion strategies11. In PROCTOR, over 60 % of CTO lesions (i.e. 50 % of all treated native vessel lesions) were completed retrogradely. Second, high-risk (occluded, thrombotic, aneurysmal, friable and unsuitably diseased) SVG’s, which carry the greatest likelihood of embolisation, no reflow and therefore peri-procedural MI were excluded from the study. These two observations may in part explain the disproportionate peri-procedural MI rates between cohorts (1 % versus 13 %), which acted as a primary driver for the significant difference in composite MACE in favour of SVG PCI (Table 3). Of note, the non-fatal target coronary territory MI at > 48 hours was identical between the cohorts.
Table 3: Comparison of the % CTO lesions and MACE across datasets
Third, a four-fold difference in stent length, 23 mm and 84 mm in the SVG PCI and native vessel PCI cohorts respectively was observed. Calcification, long lesions, negative remodelling, tortuosity and hinge-points (all prevalent characteristics of post-CABG native vessel lesions, the RCA in particular) predict stent failure and MACE. Additionally, stent undersizing and malapposition is also higher following CTO PCI secondary to vessel growth post revascularisation. In such complex lesion cohorts, intravascular imaging guidance and optimisation has been shown in numerous randomised trials and meta-analyses to reduce cardiac death and MACE. It now carries a Class IA guideline recommendation for use in complex lesions. However, in PROCTOR, the intravascular imaging rate was low at 23 % (data kindly shared by authors), rendering the observation of increased clinically driven TLR in the native PCI cohort less surprising.
Fourth, whilst there is no randomised data supporting the benefit of SVG coiling following native vessel revascularisation, recent observational data demonstrated persistent graft patency to be an independent predictor of CTO failure at 1 year12. In PROCTOR, SVG’s were not occluded and whether this had a negative impact on the 12-month native vessel composite MACE cannot be directly measured but remains a possibility.
The equivalent technical success rate of 93 % in a highly complex native vessel cohort is extremely impressive and a direct reflection of the expertise and skillset of the recruiting operators. However, this is not generalisable to the wider PCI community.
Recognising all the above, the benefits of SVG PCI are multiple including: accessibility to all PCI operators given no requirement for advanced skillsets; lower risk of procedural complications; shorter procedural time; lower radiation dose; single access reducing vascular complication risk; reduced contrast load; and an easier overall experience for the patient.
Finally, based on existing data demonstrating cumulative and incremental increase in SVG failure with time, some convergence of the Kaplan–Meier curves is perhaps expected with longer-term follow-up. The 3– and 5–year outcomes will therefore be crucial to understanding PCI durability across the two cohorts.
Conclusion
PROCTOR provides the first randomised evidence demonstrating that in appropriately selected, non-high-risk SVGs there is a clear benefit to graft over native vessel revascularisation at 12 months, with reduction in MACE driven by peri-procedural MI and CD-TLR. Whether this data evokes a down-grading for post-CABG native vessel PCI, remains to be seen.
References
- Neumann, F. J. et al. 2018 ESC/EACTS Guidelines on myocardial revascularisation. EuroIntervention. 2019 Feb 20;14(14):1435-1534.
- Back, L. & Ladwiniec, A. Saphenous Vein Graft Failure: Current Challenges and a Review of the Contemporary Percutaneous Options for Management. J Clin Med. 2023 Nov 15;12(22):7118
- Xenogiannis, I. et al. Saphenous Vein Graft Failure: From Pathophysiology to Prevention and Treatment Strategies. Circulation. 2021 Aug 31;144(9):728-745.
- Goldman, S. et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004 Dec 7;44(11):2149-56.
- Pereg, D. et al. Native coronary artery patency after coronary artery bypass surgery. JACC Cardiovasc Interv. 2014 Jul;7(7):761-7.
- Lawton JS et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e4-e17.
- De Winter, R. W. et al. Percutaneous coronary intervention of native coronary artery versus saphenous vein graft in patients with prior coronary artery bypass graft surgery: Rationale and design of the multicenter, randomized PROCTOR trial. Am Heart J. 2023 Mar:257:20-29.
- De Winter, R. W. et al. PCI of Native Coronary Artery vs Saphenous Vein Graft After Prior Bypass Surgery. J Am Coll Cardiol. 2025 Oct 28:S0735-1097(25)09477-X.
- Beerkens FJ et al. Native coronary artery or bypass graft percutaneous coronary intervention in patients after previous coronary artery bypass surgery: A large nationwide analysis from the Netherlands Heart Registration. Int J Cardiol. 2024 Jun 15:405:131974.
- Brilakis ES et al. Percutaneous Coronary Intervention in Native Coronary Arteries Versus Bypass Grafts in Patients With Prior Coronary Artery Bypass Graft Surgery: Insights From the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. JACC Cardiovasc Interv. 2016 May 9;9(9):884-93.
- Toma A et al. Clinical implications of periprocedural myocardial injury in patients undergoing percutaneous coronary intervention for chronic total occlusion: role of antegrade and retrograde crossing techniques. EuroIntervention. 2018 Apr 20;13(17):2051-2059.
- Poletti E et al. Impact of Postprocedural Graft Flow on Outcomes Following Chronic Total Occlusion Intervention in Postcoronary Artery Bypass Graft Patients: A Detailed Angiographic Analysis. Am J Cardiol. 2024 Sep 1:226:24-33.
Authors





No comments yet!