INFINITY-SWEDEHEART - Percutaneous coronary intervention with a bioadaptor compared to a contemporary drug eluting stent- one year primary outcomes
Reported from ESC Congress 2024
Daniele Giacoppo provides his take on the main results of the INFINITY-SWEDEHEART trial presented by David Erlinge at the ESC Congress 2024 in London.
Why this study? – The rationale/objective
In recent decades, drug-eluting stents have been the standard device for percutaneous coronary interventions. Although contemporary drug-eluting stents provide high anti-restenotic effectiveness and biocompatibility, the permanent metallic implants lock the target coronary artery segment in a cage precluding positive adaptive remodelling and natural pulsatility. Variable proportions of target lesion failure after treatment with second-generation DESs may be associated with this vascular loss of function and long-term data on second-generation DESs showed that after the first year following percutaneous coronary intervention, major adverse cardiac events occur at a constant incidence of 2-3% per year.
The DynamX bioadaptor is a novel sirolimus-eluting, thin-strut (71 µm) cobalt-chromium platform equipped with helical strands that unlock and separate after degradation of the bioresorbable polymer coating. This technical feature aims to promote compensatory adaptive vessel remodelling and restore normal vascular motion.
The INFINITY-SWEDEHEART trial sought to evaluate the long-term efficacy and safety of the DynamX bioadaptor compared to a contemporary drug-eluting stent for the treatment of coronary artery disease.
How was it executed? – The methodology
INFINITY-SWEDEHEART was a single-blind, registry-based, randomized clinical trial conducted at 20 centres in Sweden, in which patients undergoing percutaneous coronary intervention for chronic or acute coronary syndromes were assigned in a 1:1 ratio to receive either the DynamX bioadaptor or the Resolute Onyx zotarolimus-eluting stent (www.clinicaltrial.gov: NCT04192747).
The trial was primarily designed to demonstrate the noninferiority of the DynamX bioadaptor to the Resolute Onyx stent in terms of the primary endpoint of target lesion failure, a composite of cardiovascular death, target vessel myocardial infarction, or ischemia-driven target lesion revascularization. Assuming a 1-year event-free survival of 92.5% in the drug-eluting stent control group, an accrual period of 12 months, and a drop-out of 2%, a total of 2150 patients allowed to assess with 95% power and a margin of 4.2% the noninferiority of DynamX at an alpha of 0.025.
Nevertheless, the investigators decided to improve the required sample size to 2400 subjects. An exploratory superiority testing was prespecified if noninferiority was met. The secondary objective was to sequentially assess the superiority of the DynamX bioadaptor over the Resolute Onyx stent in terms of target lesion failure and target vessel failure over a period ranging from 6 months to 5 years since implantation.
Key inclusion criteria included successful predilation of ≥1 target lesion(s), ≤3 target lesions requiring treatment during the index procedure, ≤2 target lesions and 1 non-target lesion requiring treatment during the index procedure, distance between multiple lesions ensuring a gap of ≥10 mm between study devices, and target lesion vessel diameter and length amenable to treatment with both devices. Key exclusion criteria included acute heart failure, history of chronic heart failure with left ventricular ejection fraction <30%, advanced renal disease, recent (≤12 months) percutaneous coronary intervention, left main disease, venous or arterial bypass grafts as target vessels, in-stent restenosis, chronic total occlusion, ostial (<3 mm) lesion, severe calcification, severe tortuosity, and bifurcation disease requiring a 2-stent strategy.
After percutaneous coronary intervention, unless there were contraindications, a clopidogrel-based dual antiplatelet therapy for at least 6 months was recommended in patients with chronic coronary syndrome and a ticagrelor- or prasugrel-based dual antiplatelet platelet therapy for at least 12 months in patients with acute coronary syndrome.
The events were independently adjudicated by a clinical event committee and a core laboratory, with oversight provided by a data safety monitoring board. The trial was funded by the DynamX bioadaptor manufacturer.
What is the main result?
In the INFINITY-SWEDEHEART trial, 1201 patients (1419 lesions) were randomly assigned to DynamX and 1198 patients (1431 lesions) were randomly assigned to Resolute Onyx. The mean age of patients was 68.2 years, 24.0% were female, 17.9% had diabetes, 59.7% had hypertension, 43.2% had hyperlipidemia, 14.6 were current smokers, 11.9% had previously experienced a myocardial infarction, and 14.2% had previously undergone percutaneous coronary intervention. Most of the patients showed 1-vessel disease (83.2%), more often in the left anterior descending (51.0%), the average lesion length was 24.6 mm, and the average reference vessel diameter 3.2 mm. Successful predilation was achieved in 99.8% of lesions and postdilation was performed in 58.5% of the lesions assigned to DynamX vs. 52.9% of the lesions assigned to Resolute Onyx.
Procedural and device success in the DynamX and Resolute Onyx groups was very high (99.5% vs 99.8% and 99.6% vs 99.9%), without statistically significant differences.
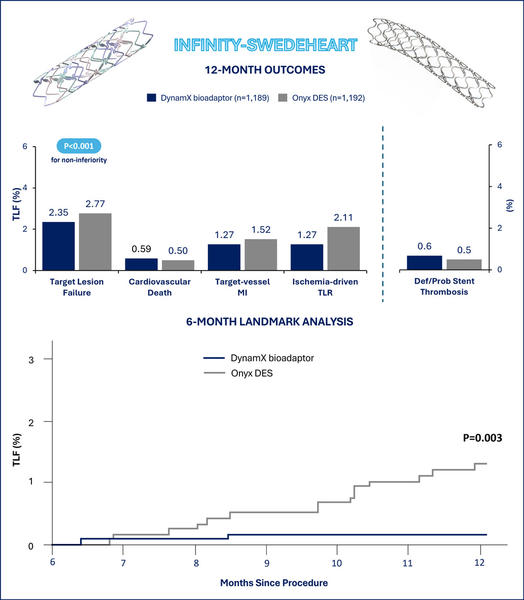
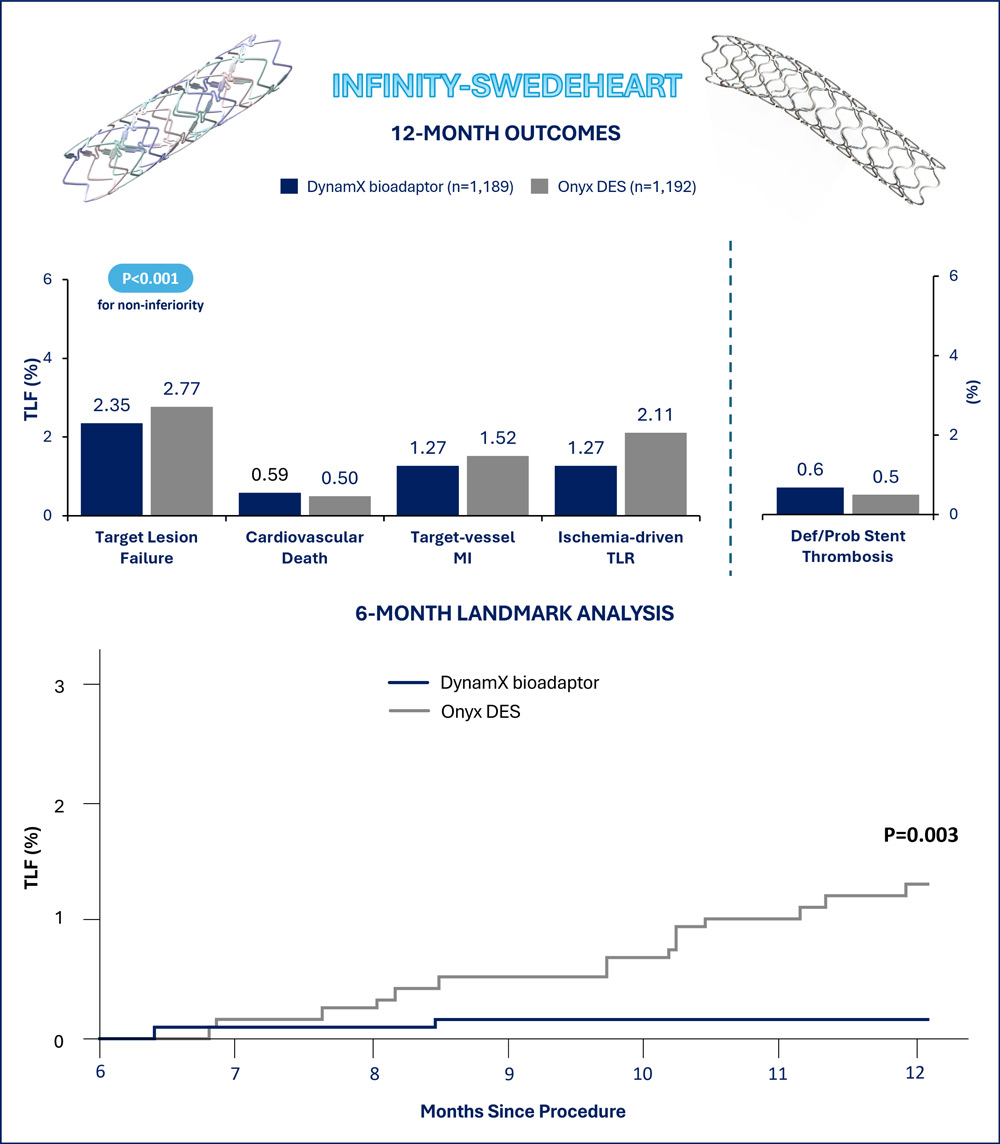
At 1 year, 2.35% of the patients assigned to DynamiX and 2.77% of the patients assigned to Resolute Onyx experienced target lesion failure. The absolute risk difference met the prespecified noninferiority margin (0.41%, 95% CI -1.94 to 1.11%, pnoninferiority<0.001). In the exploratory testing for superiority, the difference between the two treatment groups was not significant.
At 1 year, no significant differences in the target lesion failure individual components of cardiovascular death (0.59% vs 0.50%), target-vessel myocardial infarction (1.27% vs 1.52%), and ischemia-driven target lesion revascularization (1.27% vs 2.11%) were observed between the DynamX and Resolute Onyx groups. Consistently, target vessel failure (3.03% vs 3.52%) and ischemia-driven target vessel revascularization (2.03% vs 2.86%) were not significantly different between groups.
In the complementary 6-month landmark analysis, the DynamX group showed a significant reduction in target lesion failure compared with the Resolute Onyx group (0.2% vs. 1.3%, P=0.003), essentially driven by reductions in target vessel myocardial infarction (P=0.012) and ischemia-driven target lesion revascularization (P=0.003). The analysis of target vessel failure after 6 months since the index percutaneous coronary intervention showed consistent results (0.6% vs. 1.8%, P=0.008).
Critical reading and the relevance for clinical practice
The promising findings of INFINITY-SWEDEHEART should be cautiously interpreted for some reasons. In more detail, the authors expected a 92.5% target lesion failure-free survival at 1 year in the control group, corresponding to a cumulative incidence of 7.5%. However, the 1-year cumulative incidence of target lesion failure with the Resolute Onyx drug-eluting stent was 2.77%, thus approximately 3 times lower. This observation made the non-inferiority margin of 4.2% excessively broad as the DynamX group could experience incidences of target lesion failure about 2 times higher compared to the Resolute Onyx group and and the upper limit of the 95% confidence interval of the risk difference would probably still result in a non-inferiority.
Considering the observed control group incidence of 2.77%, the originally assumed non-inferiority margin translated into a shift of the non-inferiority hazard ratio from 1.60 to 2.57. After remodulating the assumptions on the relative scale based on the observed incidences, a total sample size of more than 5600 patients would have been required to test the two groups for non-inferiority.
The low 1-year incidences of target lesion failure observed in the two groups, on the one hand, support the substantial advances of contemporary devices and percutaneous coronary intervention techniques, on the other may indicate that on average the patients included in the INFINITY-SWEDEHEART trial had low ischemic risk profile and non-complex coronary artery disease. The latter argumentation is corroborated by the low baseline prevalences of major risk factors (diabetes, hypertension, hyperlipidemia, current smoking, prior myocardial infarction, and prior percutaneous coronary intervention) and the predominance of 1-vessel disease with lesions generally of limited extension. Importantly, granular descriptions of key anatomic and plaque characteristics that may invalidate the favourable short- and long-term performance of the DynamX bioadaptor, primarily lesion calcification and angulation, were not provided. These limitations warrant further analysis in study populations closer to those involved in the real-world practice.
The most interesting findings from INFINITY-SWEDEHEART probably come from the prespecified secondary analysis assessing the outcomes between groups after 6 months, when the DynamX bioadaptor helical strands unlock. This analysis indicated low and plateauing adverse event rates in the DynamX group compared to the Resolute Onyx group as a plausible result of the vessel motion and function restoration. Nevertheless, the absolute risk difference between groups was 1.1% and the available sample size cannot exclude a play of chance. For these reasons, further data at a more prolonged follow-up are awaited to confirm whether the effects associated with the DynamX bioadaptor are maintained over time and can translate into tangible clinical benefits over conventional drug-eluting stents.




No comments yet!