03 Sep 2025
DAPT-SHOCK-AMI trial: Cangrelor in cardiogenic shock
Reported from ESC Congress 2025
Kalaivani Mahadevan and Fizzah Choudry provide their take on DAPT-SHOCK-MI presented by Zuzana Motovska at ESC Congress 2025 in Madrid.
Background and Rationale
Acute myocardial infarction [AMI] complicated by cardiogenic shock [AMICS] occurs in 5-10 % of hospitalised AMI cases, carrying a mortality rate of 40–50 %1. Coronary reperfusion is the definitive intervention to reduce mortality, with recent randomised data supporting additional use of percutaneous micro-axial LV support device2. Exclusion of AMICS patients from landmark antiplatelet trials has left significant evidence gaps, rendering clinicians reliant on extrapolation from registries and small pharmacodynamic studies and leading to heterogeneity of care3-5.
In AMICS, the efficacy of oral agents, particularly thienopyridine inhibitors [clopidogrel/prasugrel] is undermined. It is also a strong predictor of stent thrombosis [ST], with registry data reporting rates as high as 20 % and up to a 12-fold increase versus standard PCI [Figure 1]7-8. Intravenously delivered Cangrelor offers near-instant, consistent platelet inhibition9. Observational data exists, supporting its utilisation in AMICS, with sub-analysis of IABP – SHOCK II showing significant improvement in TIMI flow grade and a trend to reduction in mortality, and analysis from the SCAAR registry demonstrating significant reduction in all-cause mortality [19 %] and MACE [18 %]10-11.
The DAPT–SHOCK–AMI study was designed to provide the first randomised evidence to address the uncertainty around the potential benefit of IV Cangrelor in AMICS12.
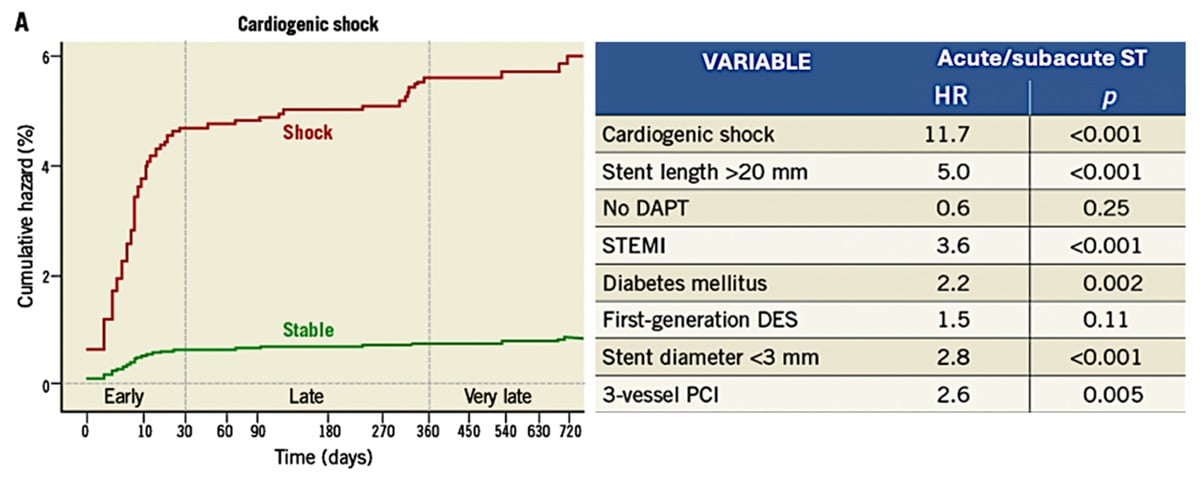
Figure 1: Cardiogenic Shock as an independent predictor for Early Acute/Subacute ST
Reference: Iqbal J, Sumaya W, Tatman V et al. EuroIntervention. 2013 May 20;9(1):62-9
Trial Design
DAPT–SHOCK–AMI is an investigator-initiated non-commercial randomised controlled, double–blind trial [RCT] comparing the use of IV Cangrelor against crushed oral Ticagrelor in AMICS.
- Key inclusion criteria: Age> 18 years, AMI indicated for emergent primary angioplasty and cardiogenic shock [pre-specified as two of systolic BP < 90 mmHg, vasopressor/inotrope requirement and signs of organ hypoperfusion]
- Key exclusion criteria: Contraindication to Ticagrelor or Cangrelor, pre-admission loading with any P2Y12 inhibitor or a pre-existing requirement for long-term anticoagulation.
Figure 2 depicts the intervention [IV Cangrelor 30 ug/kg bolus followed by 4 ug/kg infusion] and comparator [180 mg crushed oral Ticagrelor] treatment arm protocols. Identical placebo versions of both were utilised at relevant timepoints to maintain integrity of double–blinding. All patients received up-front 500mg IV Aspirin followed by 100 mg OD orally. Platelet reactivity sub-study [VASP] was undertaken to assess antiplatelet effectiveness of each P2Y12 inhibitor12.
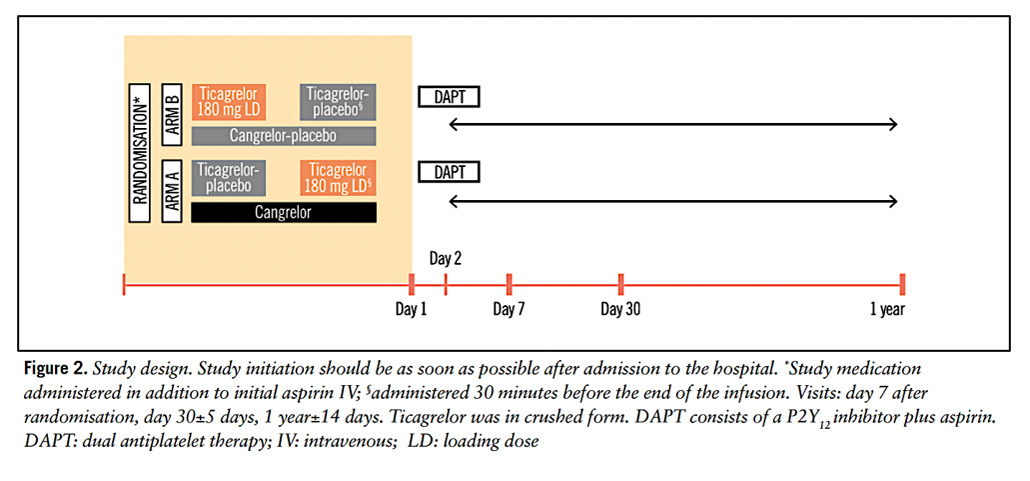
Figure 2: Treatment Arm Protocols
Courtesy of Motovska Z et al. EuroIntervention. 2024 Oct 21;20(20):e1309-e1318
Table 1 summarises pre-specified primary and secondary clinical endpoints. The primary clinical endpoint was a composite of death, MI or CVA at 30 days.
Based on a 12 % event rate difference between intervention and control arms, a required power of 80 %, a significance level of 5 % and allowing for a 3 % drop-out, a minimal sample of 550 patients was required to reject H. A non-inferiority margin of 1 % was utilised for H1 based on a 10 % event rate difference and at same power and significance, required a minimal sample of 505 patients. One-sided Z-test was performed for non-inferiority testing, where if significant, superiority was subsequently tested with a two-sided Chi2-test, alpha = 0.05. All statistical analyses were undertaken on an intention-to-treat basis, with per-protocol sensitivity analysis for secondary confirmation.
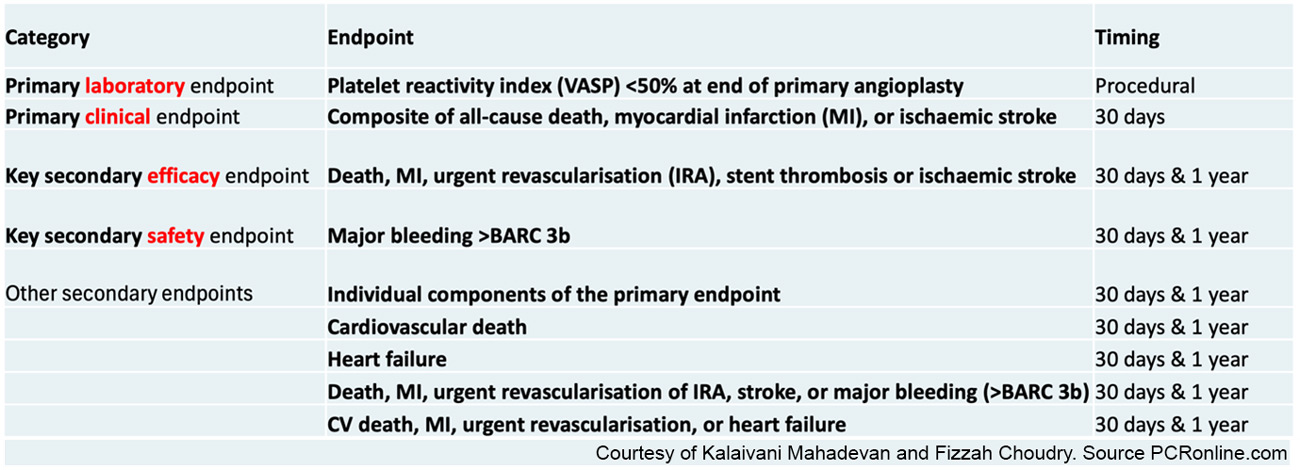
Table 1 – Primary and Secondary Clinical Endpoints
Courtesy of Kalaivani Mahadevan and Fizzah Choudry
Results
A total of 605 patients were randomised with well-balanced baseline characteristics and reflective of a true critically sick cardiogenic shock population [90 % on vasopressors/inotropes, 55 % with concomitant cardiac arrest and a median LVEF of 35 %]. Figure 3 summarises core patient, clinical presentation and CS demographics alongside lesion and PCI characteristics averaged across both arms.
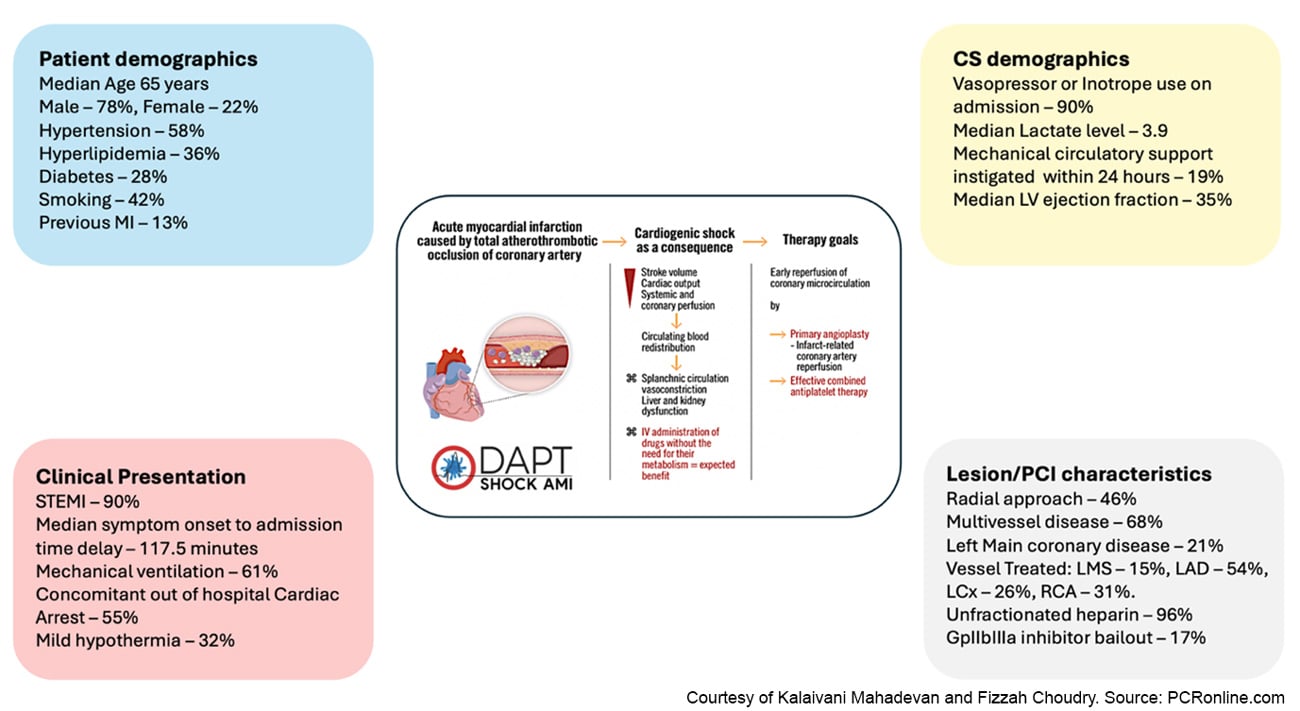
Figure 3: Patient and Procedural Characteristics
Courtesy of Kalaivani Mahadevan and Fizzah Choudry. Source: PCRonline.com
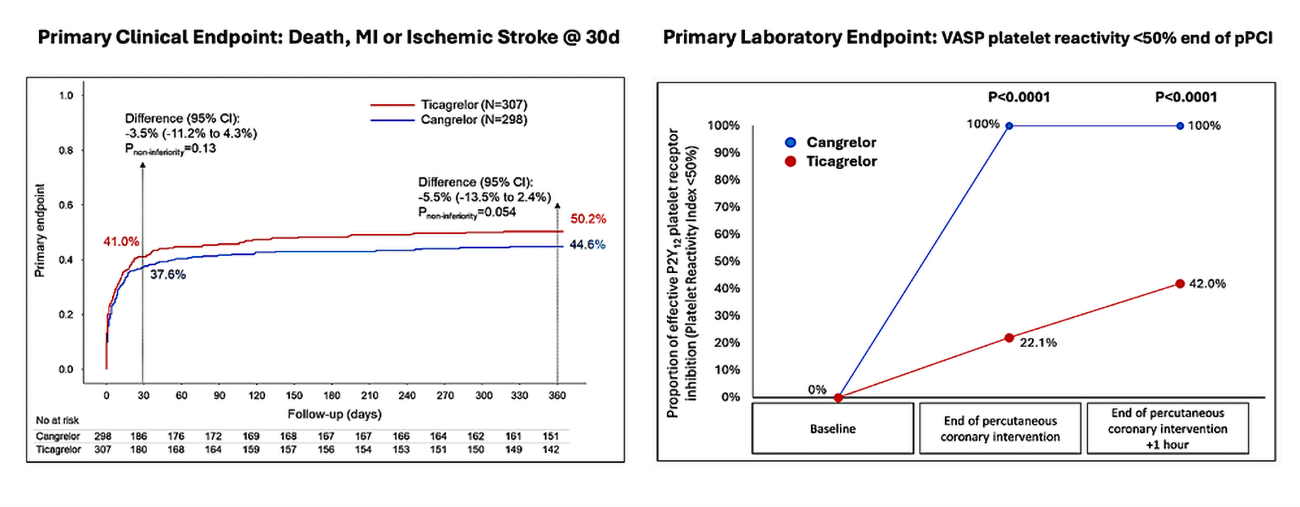
Figure 4 depicts the primary clinical and laboratory endpoint results. The clinical endpoint was not met. A total of 37.6 % and 41.0 % of patients in the Cangrelor and Ticagrelor cohorts respectively experienced death, MI or stroke, with a difference of -3.5 % [95 % CI -13.5 % to 2.4 %] and Pnon-inferiority = 0.13. The laboratory endpoint was achieved in 100 % and 22 % in the Cangrelor and Ticagrelor cohorts respectively, demonstrating superiority of Cangrelor [Psuperiority < 0.0001].
Cangrelor was also associated with improved TIMI flow grade [83 % versus 74 % with Ticagrelor, Psuperiority = 0.009] and lower rates of PCI complications [7.4 % versus 14 % with Ticagrelor, Psuperiority = 0.009] driven primarily by reduced slow/no flow and stent thrombosis. Importantly, despite increased potency of antiplatelet inhibition the secondary safety endpoint of Major BARC 33b bleeding was numerically similar between the groups [6.4 % and 5.2 % for Cangrelor and Ticagrelor respectively]. Furthermore, it also led to both reduced disability and overall healthcare costs at 12 months.
Critical Analysis and Discussion
The discussion for this review is tabulated below in Table 2 following an in-depth question-and-answer session with Zuzana Motovska, PI and study lead. With the publication pending, our purpose was to deep dive into core questions around trial methodology, results and interpretation, that readers may find interesting and informative.
What was the rationale for choosing a narrow non-inferiority margin of 1 %?
How does this compare with the non-inferiority margins used in other cardiology trials, and would a wider margin have been clinically reasonable and/or led to a different and equally credible outcome?
There has been no prior RCT of IV Cangrelor in AMICS. A strict/conservative statistical approach was utilised to ensure a positive outcome would be definitive and conclusive. In CVD trials non-inferiority margin usually ranges from 3-5 %.
From analysis, a 2 % non-inferiority margin in this study would have led to a positive outcome ie/ achievement of non-inferiority at 12 months.
The modified ITT analysis did not meet the primary endpoint at 12 months, but the Per-Protocol analysis did – is there any preliminary explanation for this?
The baseline characteristics in the mITT and PP groups were essentially identical. There was one difference – 11 patients who did not receive the recommended IV Cangrelor protocol [2 hours] with premature termination of infusion who were excluded from the PP analysis. It is possible that in this cohort IV Cangrelor did not achieve maximal effect diluting the true effect in the mITT analysis.
Was the use of CSWG SCAI shock stages [A-E] and phenotypes [non-congested, cardio-renal and cardio-metabolic] considered for use in the original trial design?
Could the phenotype help filter patients that may benefit the most from IV Cangrelor?
Pre-defined CSWG SCAI Shock stages were not utilised to define or stratify inclusion criteria. They are being assessed in sub-group analysis. The study population was reflective of a true CS population with 63.1 % and 69 % in the Cangrelor and Ticagrelor cohorts respectively, admitted in defined SCAI Stage C Shock.
Shock Phenotypes were not pre-defined for subgroup analyses but can be explored and would potentially be hypothesis-generating.
There was a 19 % use of MCS – Did this include all support devices?
Following DANGER-SHOCK with the move towards MCS in AMICS do you foresee the immediate potent action of Cangrelor as a concern for increased vascular bleeding risk/complications?
All forms of support including IABP were included. Sub-group analysis of the MCS cohort will be performed and perhaps give some signal/trend regarding the trade-off between more potent platelet inhibition and vascular complication/bleeding rates.
In a post-hoc analysis of the SCAAR registry, benefit of Cangrelor was observed in AMICS but not cardiac arrest. Has a separate sub-group analysis for AMICS without cardiac arrest been undertaken and if so, were any differences seen versus those with AMICS and cardiac arrest?
The SCAAR data analysis included patients without CS in the cardiac arrest group whilst in this study all cardiac arrest patients were in CS and all had a coronary occlusion as the cause of cardiac arrest, therefore there is some difference between the two populations. Subgroup analysis is being performed and there will be a comparator between the AMICS alone and the AMICS with cardiac arrest cohorts.
The Cangrelor arm showed higher rates of TIMI 3 flow, and fewer PCI complications potentially reflecting its early and efficacious antiplatelet effect - how might this translate to the STEMI without CS population?
IV Cangrelor reached statistical significance for superiority with respect to TIMI 3 flow grade [p = 0.009], slow/no flow [p = 0.0009] stent thrombosis [p = 0.037] and overall optimal procedural result [p = 0.014].
Use of GpIIbIIIa inhibitor was lower in the Cangrelor cohort versus the Ticagrelor cohort. This data is perhaps hypothesis generating regarding the potential benefit of IV Cangrelor in STEMI without CS.
At 12 months, was there a difference in LV recovery and the need for ICD/CRT device implantation, and could this potentially translate to a longer-term morbidity and mortality benefit and a significant healthcare cost saving?
The Echo sub-study has already demonstrated greater recovery of LV function in the Cangrelor cohort at 12 months. A cardiac MRI sub-study is on-going, assessing MVO, LV function and in the guidance of downstream device implantation. This may shed light on whether the up-front antiplatelet and procedural benefits of IV Cangrelor translate to longer-term gains in LV recovery and the reduction in need for device therapies, both of which would generate significant healthcare cost savings and improve longer-term morbidity, mortality and quality of life.
Based on these trial results, how might Cangrelor be positioned in clinical guidelines?
Overall whilst based purely on the statistical outcome, the clinical primary outcome did not achieve non-inferiority, there are strong trends and signals indicating real benefits of IV Cangrelor in this high mortality cohort – perhaps reasonable enough to consider inclusion as a IIa indication in AMICS.
The longer–term 5-year follow-up data will hopefully add strength to this.
Study Limitations
The lack of any prior RCT of antiplatelet therapy in AMICS led to a ‘best assumed’ absolute risk reduction of 12 % based on existing observational and registry data - this may not be an accurate reflection of real-world practice but was unavoidable. Patients with extreme instability or contraindications, such as active bleeding or inability to provide consent, were excluded, which whilst true of all AMICS trials does somewhat limit generalisability. The complexity of concomitant therapies, including vasopressors, mechanical circulatory support, and GP IIb/IIIa inhibitors, may partially confound the ability to fully isolate the effect of the randomized intervention. Finally, the open-label nature of background treatments, with operator discretion in their timing and use, could introduce heterogeneity across participating centres.
Conclusion
Cardiogenic Shock Trials are notoriously challenging and laborious – the authors should be congratulated on a clinically highly relevant and methodologically near flawless study design and execution in this highest mortality incurring group in CVD. Whilst the headline may simply read for many clinicians that this trial ‘did not achieve non-inferiority,’ the key to understanding the potential of the data lies in the statistical story and the granularity of the results already observed. If DAPT–SHOCK–AMI had utilised a less conservative non-inferiority margin in line with that which is widely accepted in the cardiovascular research community, non-inferiority would have been achieved at 12 months – it is therefore important not to dismiss the potential clinical and mortality benefit of IV Cangrelor. With observed divergence of curves to 12 months, whether the planned landmark 5-year blinded outcome analysis proves to be conclusive in favour of IV Cangrelor remains of significant interest. In the interim, we look forward to reading the formal publication in due course.
Aknowledgements:
With our many thanks to Professor Zuzana Motovska for her time and participation in our Q+A session for this review.
References
- Helgestad OKL, Josiassen A, Hassager C et al. Temporal trends in incidence and patient characteristics in cardiogenic shock following acute myocardial infarction from 2010 to 2017: a Danish cohort study. Eur J Heart Fail. 2019 Nov;21(11):1370-1378
- Moller JE, Engstrom T, Jensen LO et al. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N Engl J Med. 2024 Apr 18;390(15):1382-1393
- Sabatine MS, Cannon CP, Gibson CM et al. Addition of Clopidogrel to Aspirin and Fibrinolytic Therapy for Myocardial Infarction with ST-Segment Elevation. N Engl J Med 2005;352:1179-1189
- Wallentin L, Becker RC, Budaj A et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med 2009;361:1045-1057
- Wiviott SD, Braunwald E, McCabe CH et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndrome. N Engl J Med 2007;357:2001-2015.
- Naidu, S, Baran, D, Jentzer, J. et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021.. JACC. 2022 Mar, 79 (9) 933–946. https://doi.org/10.1016/j.jacc.2022.01.018
- Iqbal J, Sumaya W, Tatman V et al. Incidence and predictors of stent thrombosis: a single-centre study of 5,833 consecutive patients undergoing coronary artery stenting. EuroIntervention. 2013 May 20;9(1):62-9
- Mohammed MO, Kinnaird T, Rab ST et al. Intracoronary imaging guided percutaneous coronary intervention outcomes among individuals with cardiogenic shock. Catheter Cardiovasc Interv. 2023 Nov;102(6):1004-1011.
- Ferreiro JL, Ueno M, Angiolillo DJ et al. Cangrelor: a review on its mechanism of action and clinical development. Expert Rev Cardiovasc Ther. 2009;7:1195-201
- Droppa M, Vaduganathan M, Venkateswaran RV et al. Cangrelor in cardiogenic shock and after cardiopulmonary resuscitation: A global multicenter, matched pair analysis with oral P2Y12 inhibition from the IABP – SHOCK II Trial. Resuscitation. 2019 Apr:137:205-212
- Emilsson OK, Mohammad MA, Grimfjard P et al. Cangrelor During Percutaneous Coronary Intervention in Patients with Cardiogenic Shock or Cardiac Arrest. JACC Cardiovasc Interv. 2025 Apr 14;18(7):853-862
- Motovska Z, Hlinomaz O, Mrozek J et al. Cangrelor versus crushed ticagrelor in patients with acute myocardial infarction and cardiogenic shock: rationale and design of the randomised, double-blind DAPT-SHOCK-AMI trial. EuroIntervention. 2024 Oct 21;20(20):e1309-e1318
Latest news from ESC Congress 2025
Authors





1 comment
I APPRECIATE THE EFFORTS BY THE DAPT SHOCK AMI TRILSAIST TO THIS IMPORTANT TOPIC IN INTERVENTIONAL CARDIOLOGY.