1-year outcomes of Cardioband Tricuspid Valve Reconstruction System early feasibility study
Selected in JACC: Cardiovascular Interventions by J. Curio , G. Tang
References
Authors
William A. Gray, Sandra V. Abramson, Scott Lim, Dale Fowler, Robert L. Smith, Paul A. Grayburn, Susheel K. Kodali, Rebecca T. Hahn, Robert M. Kipperman, Konstantinos P. Koulogiannis, Mackram F. Eleid, Sorin V. Pislaru, Brian K. Whisenant, James M. McCabe, Jin Liu, Abdellaziz Dahou, Jyothy J. Puthumana, Charles J. Davidson, and on behalf of on behalf of Cardioband TR EFS Investigators
Reference
J Am Coll Cardiol Intv. 2022 Oct, 15 (19) 1921–1932
Published
15 October 2022
Link
https://www.jacc.org/doi/10.1016/j.jcin.2022.07.006Reviewers
Our Comment
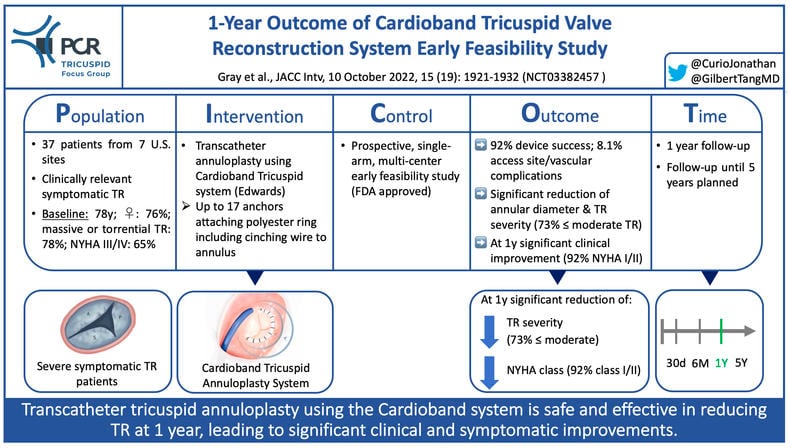
PICOT scheme highlighting key aspects of Cardioband TR EFS - Courtesy of @CurioJonathan and @GilbertTangMD, Source: PCRonline.com
Why this study – the rationale/objective?
Clinically symptomatic severe tricuspid regurgitation (TR) has been identified as an independent predictor of mortality (1). Isolated tricuspid surgery, however, also in contemporary practice remains associated with significant operative mortality of up to 12% (2). To address the resulting unmet need of patients with severe TR requiring treatment several less invasive transcatheter solutions have been developed and tested in clinical practice. The different approaches to address TR can be categorized into repair (edge-to-edge repair [T-TEER] or annuloplasty) and replacement (orthotopic or heterotopic). The Cardioband Tricuspid Valve Reconstruction System (Edwards Lifesciences) represents an annuloplasty device to reduce TR by decreasing the tricuspid annular dimensions. Up to 17 anchors attach the polyester-covered open ring-shaped implant to the annulus, followed by cinching the tricuspid valve using an enclosed contraction wire.
The Cardioband Tricuspid Valve Reconstruction System Early Feasibility study (NCT03382457) aimed to investigate the safety and performance of the device in patients with ≥ severe functional TR.
How was it executed – the methodology?
This prospective, single-arm, multi-center early feasibility study at 7 U.S. sites included 37 patients with ≥ severe functional TR to undergo transcatheter treatment using the Cardioband.
Assessed endpoints were:
- Device success (achievable placement), procedural success (defined as 30% TR EROA reduction), and clinical success (procedural success without MAEs at 30 days)
- Reduction in TR severity and tricuspid valve remodeling as well as clinical outcomes (mortality, quality of life, and MAEs), recorded up to 1 year after the procedure
What is the main result?
Seventy-six per cent of patients were women, and mean age was 77.5 ± 7.5 years. All patients suffered from at least severe TR (19% massive, 58% torrential). 65% were in NYHA functional class III/IV.
- Device success was 92% (2 cases of anchor disengagement, 1 case with inability to advance device), procedural success (at least 30% TR EROA reduction) was achieved in 83% and clinical success with freedom from MAEs at 30 days was achievable in 59%; major access site/vascular complications occurred in 8.1%
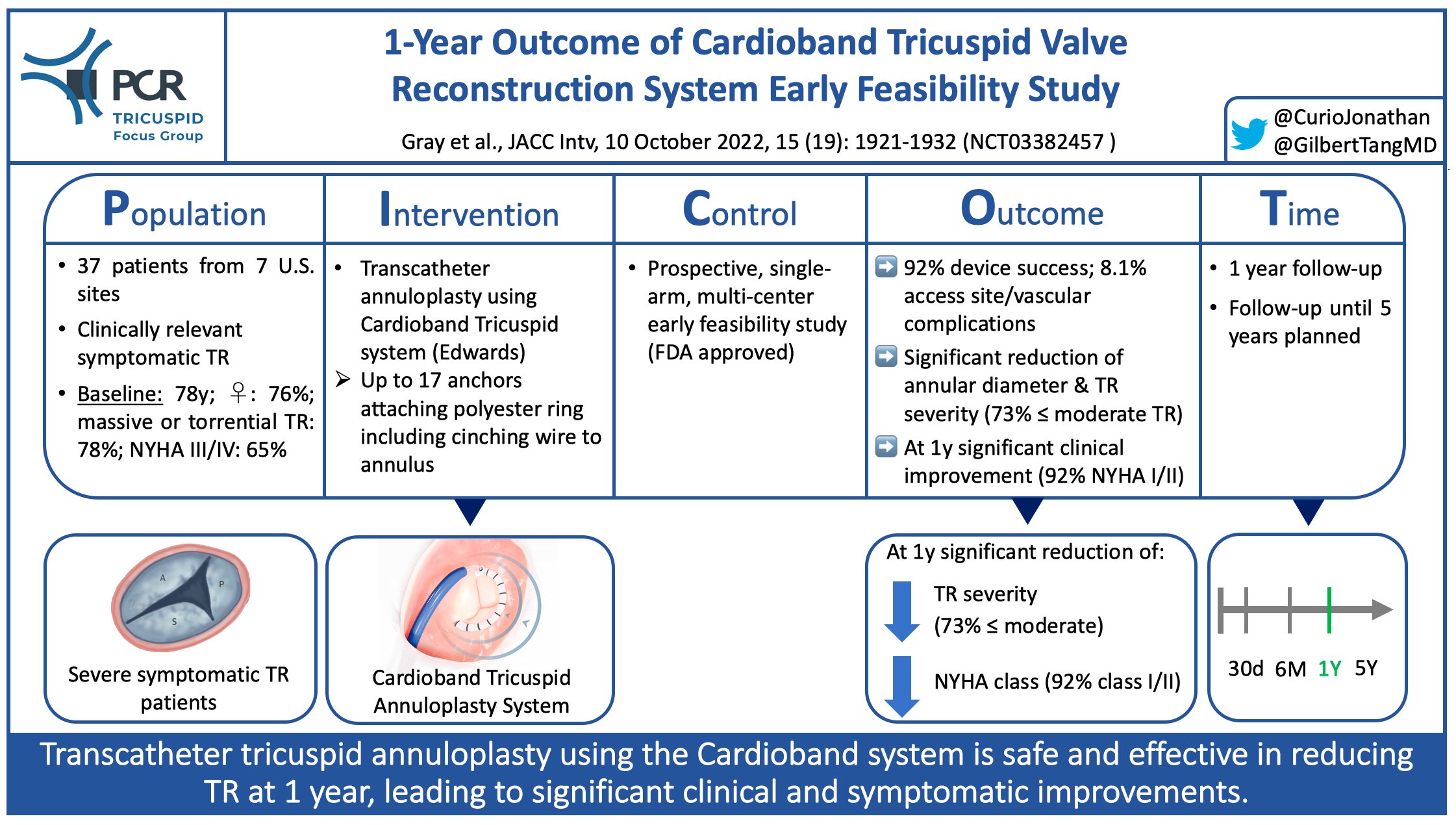
- In paired analysis after 1 year, end-diastolic septolateral tricuspid annular diameter was significantly reduced (from 4.5mm to 3.5mm, p<0.0001), resulting in significantly reduced TR severity (73% ≤ moderate TR; all patients ≥ 1 grade reduction, 73% ≥ 2 grades reduction)
- At 1 year NYHA functional class had significantly improved (92% in NYHA class I/II), and KCCQ quality of life score improved by 19 points (p<0.0001)
- All-cause mortality at 1 year was 13.5%, cardiovascular mortality was 8.1%, and rate of heart failure rehospitalisations was 10.8%
Source: JACC: Cardiovascular Interventions
Critical reading and relevance for clinical practice
The Cardioband Tricuspid annuloplasty system, following positive results of the TRI-REPAIR study (at 1 year: 63% ≤ moderate TR, 78% NYHA class I/II), in 2018 was the first transcatheter device for TR treatment to receive a CE mark (3). The now-published 1-year results of the FDA-approved Cardioband Early Feasibility Study compare well with the TRI-REPAIR data, given even higher percentages of patients with ≤ moderate TR (73%) and NYHA class I/II (92%). Of note, the effective reduction of annular dimensions and TR severity seen at discharge further improved over the course of follow-up, indicating a process of positive remodeling initiated by Cardioband implantation.
As noted, several transcatheter approaches to treat TR have been developed and a distinct evaluation of clinical as well as anatomic parameters is key to enabling proper personalized device selection, ensuring the best achievable outcomes. Especially, in late stages of TR when large coaptation gaps or leaflet tethering impair the potential effectiveness of T-TEER, annuloplasty may represent an effective treatment, addressing annular dilation as one of the key pathological mechanisms in secondary TR. A further advantage of an annuloplasty approach is that it leaves the option of other subsequent therapeutic approaches open, be it T-TEER or valve replacement.
All-cause mortality (13.5%) and cardiovascular mortality (8.1%) in this trial were higher compared to 1-year data of T-TEER as seen in the TRILUMINATE study (7.1% all-cause and 4.8% cardiovascular mortality) (4). However, the complex selection process to include patients in these trials likely lead to a bias and heterogeneous populations, at this point hindering a direct comparison of such outcomes.
The reported median procedural time of over 3 hours is notably higher than what is seen in current T-TEER practice. European real-world post-market data of the Cardioband, however, has shown that with increasing operator experience procedural time can be markedly reduced, being over 100 minutes shorter when 10 or more procedures have been performed (5). Furthermore, a new and improved Cardioband system featuring fewer anchors is currently under development, and procedure time will likely be reduced with improved ease of use.
In conclusion, given the clinical as well as anatomical heterogeneity of TR patients, there will be no “one-size-fits-all” solution, and thus, it is promising to see that annuloplasty using the Cardioband, besides T-TEER, appears to provide a reliable and effective treatment option at 1 year.
References:
- Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405-9.
- Scotti A, Sturla M, Granada JF et al. Outcomes of isolated tricuspid valve replacement: a systematic review and meta-analysis of 5,316 patients from 35 studies. EuroIntervention 2022.
- Nickenig G, Weber M, Schüler R et al. Tricuspid valve repair with the Cardioband system: two-year outcomes of the multicentre, prospective TRI-REPAIR study. EuroIntervention 2021;16:e1264-e1271.
- Lurz P, Stephan von Bardeleben R, Weber M et al. Transcatheter Edge-to-Edge Repair for Treatment of Tricuspid Regurgitation. J Am Coll Cardiol 2021;77:229-239.
- Körber MI, Landendinger M, Gerçek M et al. Transcatheter Treatment of Secondary Tricuspid Regurgitation With Direct Annuloplasty: Results From a Multicenter Real-World Experience. Circ Cardiovasc Interv 2021;14:e010019.






No comments yet!