15 Feb 2023
Renal denervation in the management of hypertension in adults: Consensus Document
Selected in EuroIntervention Journal by S. Brugaletta
A clinical consensus statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). The current position paper reviews the evidence for the safety and efficacy of renal denervation (RDN), summarizes aspects of the Expert Group’s discussion, and provides consensus statements for patient selection, centre requirements, procedural aspects, and considerations for future trial designs.
References
Authors
Emanuele Barbato, Michel Azizi, Roland E. Schmieder, Lucas Lauder, Michael Böhm, Sofie Brouwers, Rosa Maria Bruno, Dariusz Dudek, Thomas Kahan, David E. Kandzari, Thomas F. Lüscher, Gianfranco Parati, Atul Pathak, Flavio L. Ribichini, Markus P. Schlaich, Andrew S.P. Sharp, Isabella Sudano, Massimo Volpe, Costas Tsioufis, William Wijns, Felix Mahfoud
Reference
EuroIntervention 2023;18. DOI: 10.4244/EIJ-D-22-00723
Published
15 February 2023
Link
Read the abstract
Reviewer
My Comment
Why this study – the rationale/objective?
Since the publication of the 2018 ESC/ESH Guidelines for the Management of Arterial hypertension, several high-quality studies, including randomised, sham-controlled trials, on catheter-based renal denervation (RDN) were published, confirming both the blood pressure (BP)-lowering efficacy and safety of radiofrequency and ultrasound RDN in a broad range of patients with hypertension, including resistant hypertension.
For this reason, a Clinical Consensus Document by the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular (CV) Interventions (EAPCI) on RDN in the management of hypertension was considered necessary to inform clinical practice.
How was it executed? - the methodology
The current position paper reviews the evidence for the safety and efficacy of RDN, summarizes aspects of the Expert Group’s discussion, and provides consensus statements for patient selection, centre requirements, procedural aspects, and considerations for future trial designs.
In controversial areas, a consensus was achieved by agreement of the expert panel after detailed discussions.
The working group members of this document were equally selected by the ESC Council on Hypertension and the EAPCI.
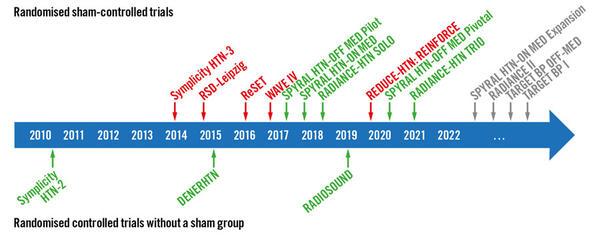
Figure. Landmark RDN trials. Overview of important randomised controlled trials with (top) and without (bottom) an invasive sham-control group. Green indicates that the trial met its primary efficacy outcome; red indicates that the trial did not meet its primary efficacy outcome.
Source: EuroIntervention Journal
What is the main result?
This document has several parts analyzing the various aspects of RDN.
- In the first part, there is a review of the clinical data available, discussing in particular RDN efficacy or safety. Specifically, data from registries and sham-controlled trials indicate a sustained blood pressure-lowering effect of RDN for up to three years. Long-term follow-up data up to three years did not reveal any significant increase in de novo renal artery stenosis (< 1 %) or worsening kidney function beyond the expected rates in hypertensive patients with normal or mild-to-moderately reduced kidney function.
- According to the available evidence, this expert group suggests considering RDN in patients with uncontrolled hypertension despite treatment with ≥ 3 antihypertensive drugs in appropriate doses, including a diuretic, confirmed by an out-of-office blood pressure (BP) measurement, preferably ambulatory BP measurement, (i.e. resistant hypertension) and an eGFR ≥ 40 ml/min/1.73 m2. It is also strongly advised to exclude secondary causes of hypertension before RDN is considered.
- RDN may be a possible treatment option in patients unable to tolerate antihypertensive drugs in the long term and express a preference to undergo RDN in a tailored shared decision-making process.
- Multidisciplinary hypertension teams involving experts on hypertension and percutaneous CV interventions should evaluate the indication and perform RDN. Standard operating procedures are suggested for each device to achieve the most effective renal nerve ablation in optimal periprocedural patient security conditions.
- Future research is needed to address open questions and investigate the impact of BP-lowering with RDN on clinical outcomes and potential clinical indications beyond hypertension. For devices approved in certain indications, allocating patients to a sham procedure can be avoided. Comparisons with an active comparator, for example, an already approved device (or drug therapy), could be an alternative.
Critical reading and the relevance for clinical practice
After the initial interest about RDN from the interventional cardiology community some years ago, neutral data from studies have reduced the interest about it. Since that, new data coming from well-conducted trials have been presented, showing a sustained long-term efficacy and safety of the procedure. Nevertheless, these new data have faced barriers in making RDN of interest again for our community. In this regard, the present document makes a summary of data available, showing that RDN is indeed a well-positioned technology for the treatment of arterial hypertension, and provides a consensus statement for standardizing the procedure, by selecting the patient who may benefit the most and by providing the minimum each center should have to start such program.
There are many interesting points to be considered. First of all, the document per se represents a good start for expanding use of RDN, by highlighting its benefits and limitations. RDN should be indeed considered as an adjunct treatment option in uncontrolled resistant hypertension confirmed by ambulatory BP measurements despite best efforts at lifestyle and pharmacological interventions. RDN may also be used in patients who are unable to tolerate antihypertensive medications in the long term.
Second, it is of note to read that a shared decision-making process is a key feature and should include the preference of a well-informed patient on the benefits and limitations of the procedure: it may be the time to have a specific multidisciplinary team - involving hypertension experts and interventionalists - besides the heart and valve team to evaluate the indication and facilitate the RDN procedure. The decision-making process should take (i) the patient’s global CV risk and/or (ii) the presence of hypertension- mediated organ damage or CV complications into account.
Last, but not least, interventionalists require expertise in renal interventions, and specific training in RDN procedures, as well as centers performing these procedures require the skills and resources to deal with potential complications.
It is time to start again thinking about this procedure, which is underused in many countries. Future research is also needed to address open questions and investigate the impact of BP-lowering with RDN on clinical outcomes and potential clinical indications beyond hypertension.