30 Mar 2023
Coronary obstruction from TAVR in native aortic stenosis : development and validation of multivariate prediction model
Selected in JACC: Cardiovascular Interventions by A. Beneduce , J. Curio , N. Dumonteil
This study aimed to develop a multivariable prediction model to estimate the risk of CO after TAVI in native anatomy based on the largest available pre-procedural CT dataset.
References
Authors
Jaffar M. Khan, Norihiko Kamioka, John C. Lisko, Emily Perdoncin, Cheng Zhang, Aneel Maini, Mao Chen, Yijian Li, Sebastian Ludwig, Dirk Westermann, Ignacio J. Amat Santos, Łukasz Kalinczuk, Jan-Malte Sinning, Tomohiro Kawaguchi, Yasushi Fuku, Asim N. Cheema, Afonso Félix-Oliveira, Masanori Yamamoto, Ai Kagase, Pablo Codner, Raquel del Valle, Vijay S. Iyer, Hyo-Soo Kim, Mao-Shin Lin, Brijeshwar Maini, Roberto Rodriguez, Matteo Montorfano, Marco B. Ancona, Norio Tada, Masaki Miyasaka, Hasan Ahmad, Nicholas J. Ruggiero, Rebecca Torguson, Itsik Ben-Dor, Christian C. Shults, Gaby Weissman, Robert J. Lederman, Adam B. Greenbaum, Vasilis C. Babaliaros, Ron Waksman, Toby Rogers
Reference
JACC Cardiovasc Interv. 2023 Feb; 16(4):415-425
Published
27 February 2023
Link
Read the abstract
Reviewers
Our Comment
Why this study – the rationale/objective?
Following positive data from randomized trials across the spectrum of surgical risk, transcatheter aortic valve implantation (TAVI) has become a mainstay of aortic stenosis (AS) treatment1. Thanks to technological and procedural refinements, the rate of procedural complications has substantially declined compared to earlier days, making TAVI a generally safe and streamlined intervention. However, specific procedural risks still necessitate distinct attention. Here, coronary obstruction (CO), although rare (< 1 %), represents a life-threatening complication, bearing a very high mortality burden of over 40 % at 30-days after the procedure.
Precise preprocedural planning with careful analysis of patient’s anatomy is paramount in preventing CO. However, current evidence supporting anatomical risk factors for CO is limited2. Thus, the present study by Khan et al. aimed to develop a dedicated multivariable prediction model to estimate the risk of CO after TAVI in native anatomy based on the largest available pre-procedural computed-tomography (CT) dataset.
How was it executed? - the methodology
Overall, 60 patients with angiographically confirmed obstruction at one or both coronary arteries after TAVI were enrolled from the CO-TAVR (Coronary Obstruction with Transcatheter Aortic Valve Replacement) and COBRA (Coronary Obstruction Risk Assessment) registries. On the other hand, 1,381 TAVI cases without CO from the MedStar Aortic Valve Database formed the control group.
- Pre-procedural multidetector CT images were analyzed for annulus dimensions, coronary height, sinus of Valsalva dimensions, cusps calcification volume, as well as height and width of the sinotubular-junction. Valve-to-coronary (VTC) measurements were obtained after transcatheter heart valve (THV) implant simulation.
- Predictors of obstruction were identified by multivariate regression.
- The prediction model was internally validated against a propensity-matched cohort of 60 patients without CO from the control group.
What is the main result?
- CO carried a significantly higher risk of in-hospital death (26.7 % vs 0.7 %; p < 0.001).
- CO affected more commonly the left coronary artery (78.3 %, 16.7 % right, and 5.0 % both) and the most frequent mechanism was direct obstruction of the ostia by native leaflet (92.1 %).
- Coronary artery height and sinus width, but not annulus area, were significant risk factors for CO by logistic regression but performed poorly in predicting CO.
- A new multivariate prediction model was created, combining cusp height > coronary artery height AND VTC ≤ 4 mm OR culprit leaflet calcium volume ≥ 600 mm3. The model performed well for both left (sensitivity 0.93, specificity 0.84, AUC 0.93) and right coronary artery (sensitivity 0.92, specificity 0.96, AUC 0.94).
Critical reading and the relevance for clinical practice
This study reinforces the concept that CO after TAVI in native anatomy is a deadly complication, requiring accurate prediction tools, which should rely on a multiparametric anatomical assessment rather than on single measurements. Using contemporary imaging analysis software, the authors provided us with a reliable multiparametric CT-based CO prediction model. This model encompasses commonly used variables as well as new ones, such as coronary cusp height (defined as the vertical distance from the annular plane to the top of the cusp commissural attachment) and independent leaflet calcium burden.
The proposed algorithm is easy to implement in clinical practice and should be integrated in the routine evaluation of AS patients to better inform Heart Team decision making on treatment options. Identification of high-risk features of CO might eventually lead to reconsider surgery in selected low-risk patients with long life expectancy or to implement coronary protection strategies in TAVI candidates. We provide an example of the practical application of the algorithm in 3 different cases in Figure 1.
Figure 1: Practical application of the proposed algorithm for CO risk assessment after TAVI in native AS. Different clinical scenarios highlight the importance of multiparametric assessment.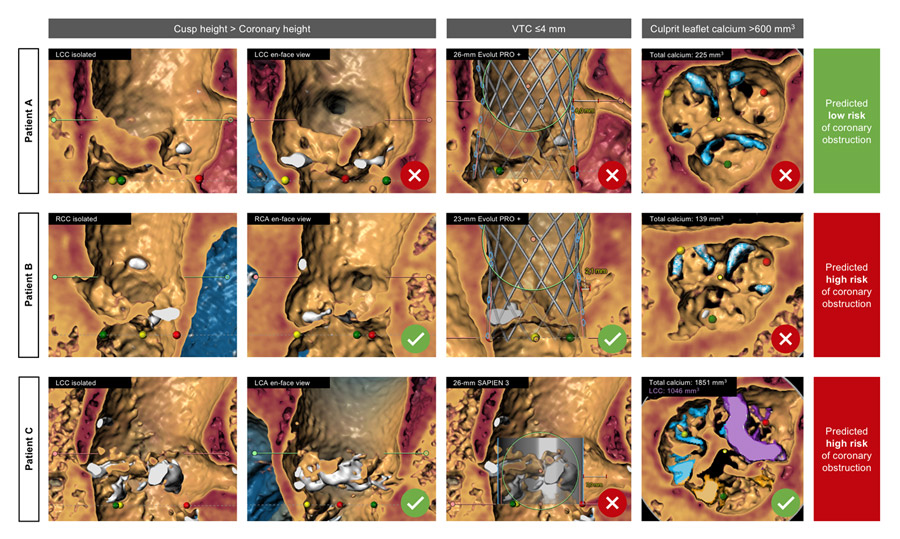
Another relevant information for daily clinical practice comes from the observation that 92.1 % of CO events in this study occurred at the level of coronary ostium, highlighting that direct CO might be the most prevalent mechanism in the setting of native TAVI. Despite the accuracy of the proposed model, it is worth noting that, in native anatomy CO, risk is also related to a dynamic behavior of the leaflets, which might be difficult to predict. This creates some gray zone situations, that might be solved by intraprocedural evaluation techniques, such as contrast injection during native valve predilatation (Video 1) or intravascular ultrasound after THV deployment (Video 2)3.
On the other hand, the predictive model might have limited ability to address the risk of indirect CO due to sinus sequestration. Beside native anatomy, this mechanism of CO is especially relevant for TAVI in failed surgical valves or redo-TAVI4. Although, some features such as the proposed coronary cusp height measurement might apply even to this context, further studies are warranted to develop a complementary prediction model for indirect CO. The next challenge will then be integrating these tools into a more complex algorithm for lifetime management planning considering the differential risk of CO even at the time of potential future reintervention.
References
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sadaba JR, Tribouilloy C, Wojakowski W, Neumann FJ, Myers P, Abdelhamid M, Achenbach S, Asteggiano R, Barili F, Borger MA, Carrel T, Collet JP, Foldager D, Habib G, Hassager C, Irs A, Iung B, Jahangiri M, Katus HA, Koskinas KC, Massberg S, Mueller CE, Nielsen JC, Pibarot P, Rakisheva A, Roffi M, Rubboli A, Shlyakhto E, Siepe M, Sitges M, Sondergaard L, Sousa-Uva M, Tarantini G, Zamorano JL, Benchabi Y, Chilingaryan A, Metzler B, Rustamova Y, Shumavets V, Lancellotti P, Smajic E, Trendafilova-Lazarova D, Samardzic J, Karakyriou M, Palecek T, Dahl JS, Meshaal MS, Palm K, Virtanen M, Bouleti C, Bakhutashvili Z, Boutsikou M, Kertész AB, Danielsen R, Topilsky Y, Golino P, Tuleutayev R, Elezi S, Kerimkulova A, Rudzitis A, Glaveckaite S, Sow R, Demarco DC, Bulatovic N, Aouad A, Van Den Brink R, Antova E, Beitnes JO, Ochala A, Ribeiras R, Vinereanu D, Irtyuga O, Ivanovic B, Simkova I, Gomez AG, Sarno G, Pedrazzini GB, Bsata W, Zakhama L, Korkmaz L, Cherniuk S, Khanji MY, Sharipov I, Baigent C, Aboyans V, Antoniou S, Arbelo E, Baumbach A, Čelutkiene J, Cikes M, Falk V, Fauchier L, Gale CP, Halvorsen S, Jaarsma T, Konradi A, Kotecha D, Landmesser U, Lewis BS, Linhart A, Løchen ML, Neubeck L, Petersen SE, Prescott E, Touyz RM, Galletti L, Hazekamp M, Licht P, Perier P, Prager R, Roessner E, Tsagakis K, Zientara A. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632.
- Ribeiro HB, Webb JG, Makkar RR, Cohen MG, Kapadia SR, Kodali S, Tamburino C, Barbanti M, Chakravarty T, Jilaihawi H, Paradis JM, De Brito FS, Cánovas SJ, Cheema AN, De Jaegere PP, Del Valle R, Chiam PTL, Moreno R, Pradas G, Ruel M, Salgado-Fernández J, Sarmento-Leite R, Toeg HD, Velianou JL, Zajarias A, Babaliaros V, Cura F, Dager AE, Manoharan G, Lerakis S, Pichard AD, Radhakrishnan S, Perin MA, Dumont E, Larose E, Pasian SG, Nombela-Franco L, Urena M, Tuzcu EM, Leon MB, Amat-Santos IJ, Leipsic J, Rodés-Cabau J. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol. 2013;62:1552–62.
- Pighi M, Lunardi M, Pesarini G, Castriota F, Venturi G, Gottin L, Scarsini R, Ferrero V, Ribichini F. Intravascular ultrasound assessment of coronary ostia following valve-in-valve transcatheter aortic valve implantation. EuroIntervention. 2021;16:1148–51.
- Ochiai T, Oakley L, Sekhon N, Komatsu I, Flint N, Kaewkes D, Yoon SH, Raschpichler M, Patel V, Tiwana R, Enta Y, Mahani S, Kim Y, Stegic J, Chakravarty T, Nakamura M, Cheng W, Makkar R. Risk of Coronary Obstruction Due to Sinus Sequestration in Redo Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2020;13:2617–27.







