Balloon-expandable vs self-expanding valves for transcatheter treatment of Sievers type 1 bicuspid aortic stenosis
Selected in JACC: Cardiovascular Interventions by M. Belmonte , N. Ryan
The AD-HOC Registry aimed to evaluate procedural success and clinical outcomes of current-generation BEVs and SEVs in treating stenotic Sievers type 1 BAV.
References
Authors
Andrea Buono, Andrea Zito, Won-Keun Kim, Tommaso Fabris, Chiara De Biase, Michele Bellamoli, Nicholas Montarello, Giuliano Costa, Mesfer Alfadhel, Ofir Koren, Simone Fezzi, Barbara Bellini, Mauro Massussi, Andrea Scotti, Lin Bai, Giulia Costa, Alessandro Mazzapicchi, Enrico Giacomin, Riccardo Gorla, Karsten Hug, Carlo Briguori, Luca Bettari, Antonio Messina, Mauro Boiago, Emmanuel Villa, Matthias Renker, Mario Garcia Gomez, Chiara Fraccaro, Maria Luisa De Rosa, Vivek Patel, Carlo Trani, Marco De Carlo, Giulia Laterra, Alessia Latini, Dario Pellegrini, Alfonso Ielasi, Ady Orbach, Uri Landes, Tobias Rheude, Luca Testa, Ignacio Amat Santos, Antonio Mangieri, Francesco Saia, Luca Favero, Mao Chen, Marianna Adamo, Azeem Latib, Anna Sonia Petronio, Matteo Montorfano, Raj R. Makkar, Darren Mylotte, Daniel J. Blackman, Francesco Burzotta, Marco Barbanti, Ole De Backer, Didier Tchètchè, Diego Maffeo, and Giuseppe Tarantini
Reference
J Am Coll Cardiol Intv. 2024 Nov, 17 (22) 2596–2608
Published
November 17, 2024
Link
Read the abstractReviewers
Our Comment
Designed by Marta Belmonte & Nicola Ryan. Source: PCRonline
Why this study – the rationale/objective?
Bicuspid aortic valve (BAV) presents significant challenges for transcatheter aortic valve replacement (TAVR) due to anatomical complexities such as calcified raphe, eccentric aortic root anatomy, and larger annulus dimensions, particularly in raphe-type BAV, which increases the risk of complications and mortality.
While advances in current-generation transcatheter heart valves (THVs) have improved outcomes in BAV patients, comparative data on balloon-expandable valves (BEVs) and self-expanding valves (SEVs) in this context remain limited to small, retrospective studies with inherent biases.
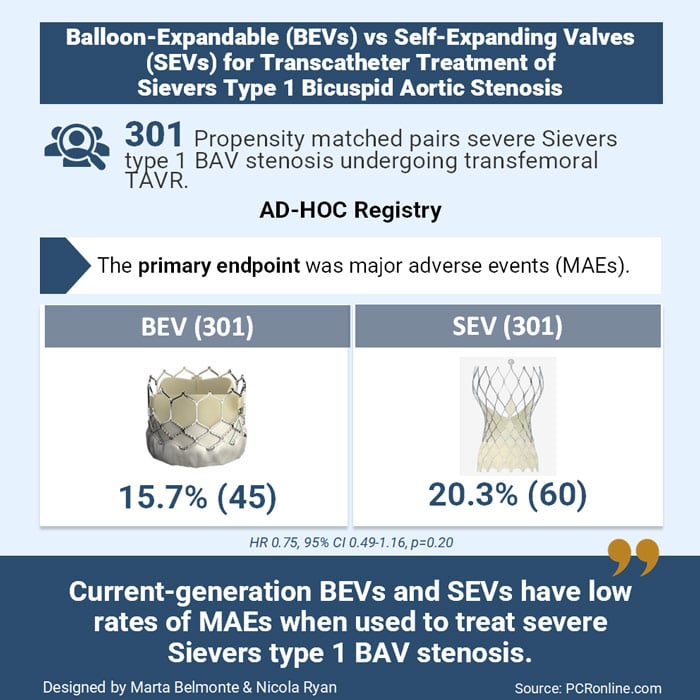
The AD-HOC Registry aimed to evaluate procedural success and clinical outcomes of current-generation BEVs and SEVs in treating stenotic Sievers type 1 BAV.
How was it executed – the methodology?
The AD-HOC Registry was a retrospective, international, multicenter study including patients with severe aortic stenosis and Sievers type 1 BAV treated with transfemoral TAVR at 24 tertiary centers.
Data on procedural outcomes, predischarge echocardiographic findings, and short-term clinical outcomes were collected at 30 days, with longer follow-up obtained through outpatient visits or phone calls. Echocardiographic and MSCT images were analysed retrospectively following current guidelines.
- The primary endpoint was major adverse events (MAEs) at follow-up, including all-cause death, neurologic events, or heart failure hospitalisation
- Secondary endpoints included technical success, 30-day device success, early safety, and their components, defined per VARC3 criteria.
What is the main result?
From January 2016 to October 2023, 979 patients were enrolled in the registry. To address baseline differences between BEV and SEV groups, a 1:1 propensity score matching (PSM) was applied, yielding 301 matched pairs. The median age was 78 years, with the majority male. The median follow-up was 1.3 years.
- The primary endpoint was comparable between BEVs and SEVs: 15.7 % vs 20.3 % (HR: 0.75; 95 % CI: 0.49-1.16; P = 0.200)
- Technical success as similar: 95.7 % vs 94.0 % (OR: 1.38; 95 % CI: 0.63-3.04; P = 0.421).
- At 30 days, BEVs were associated with a lower risk of new permanent pacemaker implantation (OR: 0.42; 95 % CI: 0.24-0.72; P = 0.002) and moderate or greater paravalvular regurgitation (OR: 0.16; 95 % CI: 0.05-0.48; P = 0.001) but a higher risk of severe patient-prosthesis mismatch (OR: 3.03; 95 % CI: 1.02-8.95; P = 0.045).
Critical reading and the relevance for clinical practice
The AD-HOC Registry provides valuable insights into the performance of current-generation THVs in treating severe Sievers type 1 BAV stenosis. Enrolling over 900 patients, it addresses important limitations of prior studies, including small sample sizes and heterogeneity.
Technical success rates for BEVs and SEVs were high (95 %), reflecting technological and procedural advancements. However, SEVs were associated with higher rates of paravalvular regurgitation (PVR) and new permanent pacemaker implantation (PPI), while BEVs had a higher incidence of severe patient-prosthesis mismatch (PPM). Notably, all BEVs had an external sealing skirt, whereas 41.2 % of SEVs did not, possibly explaining the disparities in PVR rates. Despite these differences, both devices showed comparable mid-term outcomes, including major adverse events. These results were confirmed at sensitivity analyses comparing the most commonly implanted BEV platform (SAPIEN) to the most frequently used SEV (CoreValve/Evolut, Medtronic) and comparing BEVs to the latest-generation SEV (including only SEVs equipped with an external sealing skirt).
The study highlights the need for meticulous pre-procedural planning and adaptation of sizing techniques, such as supra-annular sizing, particularly for SEVs to reduce PPI rates.
Limitations include the retrospective design, operator-dependent valve choice, lack of core lab adjudication, and absence of long-term follow-up.
Overall, while the results are encouraging, prospective studies with larger sample sizes and extended follow-up are warranted to refine device selection and optimize outcomes for this challenging patient population.







No comments yet!