PARTNER 3: transcatheter or surgical aortic-valve replacement in low-risk patients at 7 years
Selected in NEJM by I. Belluschi , S. Brugaletta
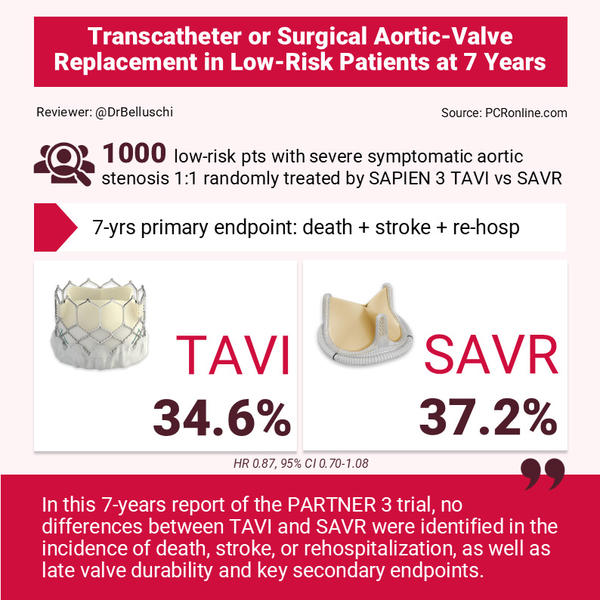
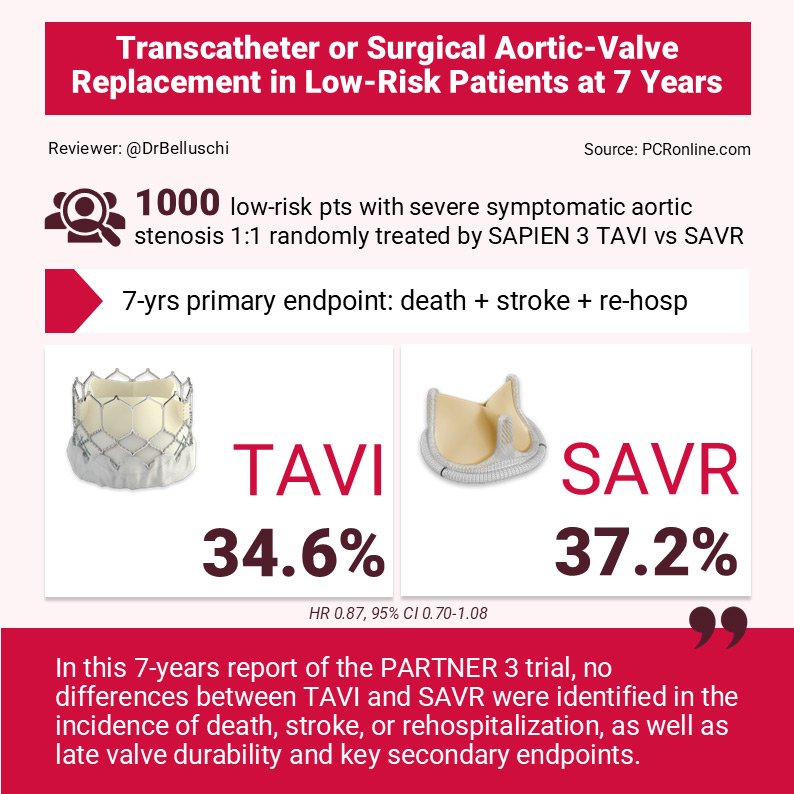
At 7 years, low-risk patients affected by severe symptomatic aortic valve stenosis 1:1 randomly treated by SAPIEN 3 TAVI or surgery showed similar clinical outcomes and late valve durability
References
Authors
Leon MB, Mack MJ, Pibarot P, Hahn RT, Thourani VH, Kodali SH, Généreux P, Kapadia SR, Cohen DJ, Pocock SJ, Zhang Y, Szerlip M, Ternacle J, Malaisrie SC, Herrmann HC, Szeto WY, Russo MJ, Babaliaros V, Nazif T, Webb JG, Makkar RR
Reference
N Engl J Med. 2025 Oct 27. doi: 10.1056/NEJMoa2509766
Published
27 October 2025
Link
Read the abstractReviewers
Our Comment
Reviewer: Igor Belluschi
Source: PCRonline.com
Why this study – the rationale/objective?
TAVI has widely become a valid alternative to surgery for the treatment of severe symptomatic aortic stenosis.
The main RCTs have shown similar clinical outcome at 1-, 2- and 5-years of follow-up.
Recent European guidelines update lowered the age cutoff for mode of intervention1, thus confirming the TAVI trend towards younger patients.
In this context, the 7-year follow-up of the PARTNER 3 trial adds consistent data on late valve durability.
How was it executed? The methodology
PARTNER 3 is a prospective, multicenter, open-label, randomised, sponsored trial in which low-risk patients (STS-PROM < 4 %) affected by symptomatic severe aortic stenosis were 1:1 randomly assigned to undergo transfemoral TAVI (balloon-expandable SAPIEN 3 valve; Edwards Lifesciences) vs. surgical aortic valve replacement with a commercially available bioprosthesis according to the operator’s preference.
Per trial protocol, patients with significant concomitant CAD underwent combined revascularisation procedures.
The two beginning primary endpoints of the trial - now analysed at 7 years - were:
- A nonhierarchical composite endpoint of death, stroke, or rehospitalisation related to the procedure/valve/heart failure (assessed by Kaplan-Meier estimates);
- A hierarchical composite endpoint of death, disabling stroke, nondisabling stroke, and the number of rehospitalisation days related to the procedure, the valve, or heart failure (assessed by the win ratio method).
All echocardiograms were evaluated at a central core laboratory and valve durability was assessed according to VARC-3 criteria.
What is the main result?
Among 1,000 patients enrolled, n = 503 were assigned to undergo TAVI and n = 497 SAVR.
Evaluation of the primary end point was performed at 7 years in 89.6 % of the patients, which showed a mean age of 73 years and a mean STS score of 1.9 %.
The first primary endpoint was similar between TAVI and surgery: 34.6 % vs 37.2 % (difference, −2.6 percentage points; 95 % CI, −9.0 to 3.7; HR 0.87, 95 % CI 0.70-1.08). In details: death, 19.5 % and 16.8 %; stroke, 8.5 % and 8.1 %; and rehospitalisation, 20.6 % and 23.5 %.
The win ratio for the second primary endpoint was 1.04 (95 % CI, 0.84-1.30).
Similarly, secondary endpoints appeared comparable as well:
- stroke (8.5 % TAVI vs 8.1 % SAVR; HR 1.00, 95 % CI 0.62-1.59);
- reintervention (6.7 % vs 6.0 %; HR 1.11, 95 % CI 0.63-1.94);
- endocarditis (2.9 % vs 2.8 %; HR 0.94, 95 % CI 0.42-2.14);
- myocardial infarction (6.0 % vs 5.6 %; HR 0.99, 95 % CI 0.56-1.75);
- major bleeding (15.6 % vs 18.5 %; HR 0.79, 95 % CI 0.57-1.09);
- new pacemaker implantation (17.3 % vs 12.8 %; HR 1.38, 95 % CI 0.97-1.97);
- clinically significant valve thrombosis (2.8 % vs 0.5 %; HR 5.70, 95 % CI 1.29-25.25); n = 7/13 TAVI thrombosis resolved with anticoagulation);
- paravalvular leak (≥ moderate 1.0 % vs 0.4 %; mild 16.7 % vs 1.6 %).
The 7-year mean transvalvular gradients assessed were 13.1 ± 8.5 mmHg after TAVI and 12.1 ± 6.3 mmHg after surgery.
Bioprosthetic valve failure was 6.9 % in the TAVI group and 7.3 % in the surgery group (HR 0.93 (0.56-1.54)).
Critical reading and the relevance for clinical practice:
In this 7-year report of the PARTNER 3 trial, no differences between TAVI and SAVR were identified in the incidence of death, stroke, or rehospitalisation, as well as late valve durability and key secondary endpoints.
Conversely to the first-year report, TAVI showed a higher trend of mortality, strokes and rehospitalisation when compared to surgery during late follow-up, despite not statistically significant. It has been postulated that the early benefit of a less-invasive procedure could be counterbalanced by an increased patient vulnerability to late adverse events in the low-risk context.
Similarly, a 5- and 7-year higher rate of spontaneous myocardial infarctions was observed after TAVI compared to surgery - maybe owed to lack of concomitant PCI - resulting in an increased incidence of late revascularisation. However, this trend could be compensated by the higher rate of early myocardial infarctions in the surgical arm, so that the final event rate between the two groups was almost comparable.
Interestingly, both groups showed equivalent low rates of valve reintervention and endocarditis and no differences were identified in the subgroup analysis.
Furthermore, mild paravalvular leak in the TAVI arm was not associated with increased mortality (HR 0.98, 95 % CI 0.57-1.38).
Regardless of a study population with a mean age of 73 years old, the similar late valve durability observed in both groups emphasises the ongoing trend of TAVI treatment in the younger population - demanding for long lasting valves - recently confirmed by the 2025 ESC/EACTS guidelines which lowered age cut-off for TAVI indication1.
Despite a higher withdrawn rate in the surgical group may have potentially biased the results, the 7-year data from the PARTNER 3 randomised trial corroborates the outcome of previous long-term TAVI reports2.
The PARTNER 3 future report is expected at 10 years and will provide more insights to help cardiologists and cardiac surgeons in the clinical decision-making process.
References
- Praz F, Borger MA, Lanz J, Marin-Cuartas M, Abreu A, Adamo M, Ajmone Marsan N, Barili F, Bonaros N, Cosyns B, De Paulis R, Gamra H, Jahangiri M, Jeppsson A, Klautz RJM, Mores B, Pérez-David E, Pöss J, Prendergast BD, Rocca B, Rossello X, Suzuki M, Thiele H, Tribouilloy CM, Wojakowski W; ESC/EACTS Scientific Document Group. 2025 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2025 Aug 29:ehaf194. doi: 10.1093/eurheartj/ehaf194. Epub ahead of print. PMID: 40878295.
- Thyregod HGH, Jørgensen TH, Ihlemann N, Steinbrüchel DA, Nissen H, Kjeldsen BJ, Petursson P, De Backer O, Olsen PS, Søndergaard L. Transcatheter or surgical aortic valve implantation: 10-year outcomes of the NOTION trial. Eur Heart J. 2024 Apr 1;45(13):1116-1124. doi: 10.1093/eurheartj/ehae043. PMID: 38321820; PMCID: PMC10984572.
Related content
For a practical interpretation of what these findings mean in daily practice, watch the PARTNER 3 interview here.







No comments yet!