Surgical left atrial appendage occlusion in VHD without AF: OPINION Trial
Reported from ESC Congress 2025
Alex Sticchi provides his take on the results of the OPINION trial presented by Yang Wang at the ESC Congress 2025 in Madrid.
Thromboembolic complications are a major concern following cardiac valve surgery, especially in patients who subsequently develop atrial fibrillation (AF). The left atrial appendage (LAA) is the predominant site of thrombus formation in AF, and its exclusion during surgery has been consistently associated with a reduction in stroke risk. Building on this evidence, the 2025 ESC/EACTS Guidelines on Valvular Heart Disease have strengthened their recommendation: surgical closure of the LAA is now a Class I, Level B intervention for patients with AF undergoing valve surgery while receiving oral anticoagulation, reflecting its established role in preventing cardioembolic events.
However, uncertainty persists for patients in sinus rhythm prior to surgery but carrying an elevated thromboembolic risk profile, as reflected by a CHA₂DS₂-VASc score ≥2. This population is clinically relevant, as postoperative AF (POAF) occurs in 15–54% of cases, and its onset is associated with both early and long-term adverse outcomes. While prophylactic closure of the LAA in these individuals could theoretically mitigate future risk, prior studies have yielded conflicting results. Against this backdrop, the OPINION trial was designed to provide high-quality randomized evidence in this specific setting.
Courtesy of Alex Sticchi. Source: PCRonline.com
Why this study – the rationale/objective?
The rationale for OPINION rested on three clinical observations. First, valve surgery patients with CHA₂DS₂-VASc ≥2 face a substantial risk of thromboembolic events, particularly when POAF occurs. Second, LAA occlusion is an established preventive strategy in AF, but its role in patients without pre-operative AF had never been rigorously tested in a large randomized controlled trial. Finally, prior observational studies and meta-analyses had offered divergent findings, some suggesting protection from stroke and others showing no clear benefit. The OPINION trial thus sought to clarify whether routine prophylactic LAA occlusion during valve surgery could reduce the composite endpoint of ischemic stroke, transient ischemic attack (TIA), and cardiovascular death at one year in patients without a history of AF.
How was it executed - the methodology
The OPINION trial was a multicentre, open-label, randomized superiority trial conducted in three cardiac surgery centres in Beijing (Fuwai Hospital, Beijing Anzhen Hospital, Beijing Chaoyang Hospital). Eligible participants were adults undergoing mitral or aortic valve surgery, in sinus rhythm, and with a CHA₂DS₂-VASc score of at least 2. Patients with prior AF/flutter, prior stroke within one month, LAA thrombus, left atrial diameter >6 cm, redo surgery, or transplant/complex congenital procedures were excluded.
A total of 2,157 patients were randomized 1:1 between April 2021 and June 2024 to SLAAO (n=1079) or no occlusion (n=1078). After exclusions and withdrawals, 2,118 patients were included in the intention-to-treat population (1062 SLAAO vs 1056 controls). Randomization was web-based and concealed until induction of anesthesia. In the SLAAO arm, the LAA was amputated and closed with a double suture; intraoperative transesophageal echocardiography (TEE) was used to verify success, and residual stumps >1 cm were corrected during the index procedure.
All patients received postoperative anticoagulation according to valve type, and were followed up at 1, 3, and 12 months, either by clinic visit or telephone. The primary endpoint was a composite of ischemic stroke, TIA, or cardiovascular mortality at one year. Secondary endpoints included each component individually, hemorrhagic stroke, BARC type III–V bleeding, postoperative AF, and AF-related healthcare utilization. The study was powered for an expected 40% risk reduction from a baseline event rate of 6.8/100 person-years, requiring ~2,118 patients for 80% power at α=0.025 (one-sided).
What are the main results?
Baseline characteristics were well balanced between groups. The mean age was 55.6 years, 32.6% were female, and the mean CHA‚ÇÇDS‚ÇÇ-VASc score was 2.87. About 54% underwent mechanical valve replacement, 29% repair, and 16% bioprosthetic replacement; 25% had concomitant CABG. The mean EuroSCORE II was 1.57%. Intraoperative metrics were comparable, with cross-clamp times of ~92 minutes and bypass times of ~123 minutes.
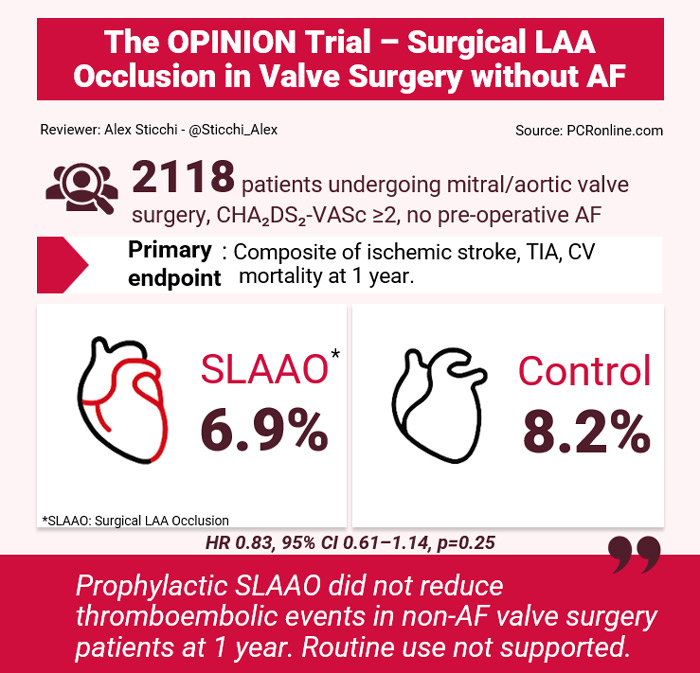
Primary endpoint: At one year, the composite outcome occurred in 6.9% (73/1062) of the SLAAO group versus 8.2% (87/1056) of the control group (HR 0.83, 95% CI 0.61-1.14; log-rank p=0.24).
Secondary outcomes:
- Ischemic stroke: identical rates of 2.5% in both arms.
- TIA: 3.9% vs 5.2% (HR 0.74, CI 0.49-1.11).
- Cardiovascular death: 0.9% vs 0.8% (HR 1.13, CI 0.43-2.92).
- POAF: 29.2% vs 32.3% (p=0.12).
- AF-related healthcare utilization: 8.8% vs 9.6% (HR 0.91).
- Safety: 30-day mortality was low (0.4% vs 0.1%), and bleeding rates (BARC IV: 1.1% vs 1.7%) did not differ significantly.
- Subgroups: No prespecified subgroup demonstrated significant interaction. Event curves suggested a divergence emerging after six months, though without statistical significance at one year.
Key Baseline Characteristics
Variable | SLAAO (n=1062) | Control (n=1056) |
|---|---|---|
Age, mean (SD) | 55.5 (11.4) | 55.6 (11.5) |
Female | 32.9% | 32.3% |
Hypertension | 46.3% | 44.1% |
Diabetes mellitus | 13.1% | 13.4% |
Previous stroke | 12.2% | 11.1% |
Rheumatic heart disease | 10.4% | 10.4% |
CHA2DS2-VASc, mean (SD) | 2.88 (0.98) | 2.87 (0.96) |
EuroSCORE II, mean (SD) | 1.58 (1.42) | 1.56 (1.28) |
Valve repair | 30.3% | 27.8% |
Mechanical replacement | 54.2% | 53.4% |
Bioprosthetic replacement | 15.2% | 18.2% |
Concomitant CABG | 25.5% | 25.8% |
Critical reading and the relevance for clinical practice
The OPINION trial is a very interesting trial because it provides the largest randomized dataset to date on prophylactic SLAAO in patients without pre-operative AF. Its design and execution were rigorous, with excellent follow-up and systematic intraoperative quality control. Nevertheless, several limitations temper its interpretation.
First, the trial was powered for a 40% relative risk reduction, which in retrospect was overly ambitious. The observed effect (~17% reduction) suggests that the study was underpowered to detect a more modest but potentially clinically meaningful benefit.
Second, the enrolled population was relatively young (mean age 55 years) and carried a lower thromboembolic burden (CHA₂DS₂-VASc ~2.9) compared to AF populations in which SLAAO has proven effective. More than half of patients underwent mechanical valve replacement, requiring lifelong anticoagulation, which may have attenuated any incremental effect of appendage occlusion.
Third, the endpoint included cardiovascular mortality, which is less directly modifiable by SLAAO and may dilute the detection of a stroke-specific signal.
Fourth, the follow-up was limited to one year. Kaplan–Meier curves suggested a potential late benefit after six months, echoing LAAOS-III, where benefit accrued over longer-term follow-up (median 3.8 years). This underlines the importance of ongoing extended follow-up in OPINION.
Finally, generalizability is limited, as nearly all patients were recruited from a single high-volume centre in China. While procedural standardization was strong, extrapolation to other healthcare systems and populations requires caution.
When compared with LAAOS-III, differences are evident. LAAOS-III enrolled older patients with AF (mean age ~71 years, CHA₂DS₂-VASc ~4.2), 77% of whom were on anticoagulation. In that higher-risk setting, SLAAO significantly reduced stroke/systemic embolism (HR 0.67, p=0.001). In OPINION, with a lower-risk, non-AF population and shorter follow-up, no clear benefit emerged.
Feature | OPINION | LAAOS-III |
|---|---|---|
Trial population | No pre-op AF; valve surgery; | AF patients; cardiac surgery |
Randomized N | 2,118 | 4,770 |
Mean age | ~55.6 y | ~71 y |
Mean CHA2DS2-VASc | ~2.9 | ~4.2 |
Oral anticoagulation at baseline | ~30% | ~76.8% |
Primary endpoint | Stroke/TIA/CV death at 1 y | Stroke/systemic embolism (median 3.8 y) |
Primary result | 6.9% vs 8.2% (HR 0.83; p=0.25) | 4.8% vs 7.0% (HR 0.67; p=0.001) |
KM curves | Non significantly separate after ~6 months | Benefit accrues over years |
Based on OPINION, routine prophylactic SLAAO cannot be recommended in patients undergoing valve surgery who do not have pre-operative AF, even if they present with CHA₂DS₂-VASc ≥2. The decision should remain individualized, taking into account valve type, anticoagulation strategy, and long-term thromboembolic risk. For now, the priority remains optimal perioperative anticoagulation and careful management of POAF.
Further studies with longer follow-up and trials focused on subgroups such as bioprosthetic or repair patients who may not require lifelong anticoagulation, are warranted.
References
- Yuan X, Ju F, Wu H, Zhao Y, Wang X, Liu S, et al. Surgical left atrial appendage occlusion in valvular heart disease without atrial fibrillation: the OPINION trial. Eur Heart J. 2025;00:1–9.
- Praz F, et al. 2025 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2025.
- Whitlock RP, Belley-Cote EP, Paparella D, et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke (LAAOS III). N Engl J Med. 2021;384:2081–91.
- Chua SK, Shyu KG, Lu MJ, et al. Clinical utility of CHADS₂ and CHA₂DS₂-VASc scoring for predicting postoperative AF after cardiac surgery. J Thorac Cardiovasc Surg. 2013;146:919–26.
- Yin L, Ling X, Zhang Y, et al. CHA₂DS₂-VASc score for identifying high risk of POAF after valve surgery: a meta-analysis. Ann Thorac Surg. 2020;109:1210–6.




No comments yet!