26 Sep 2024
Challenging aortas: how to perform TAVI after coarctation surgery?
Supported by the EuroIntervention Journal
Authors*
Giuseppe Bruschi1, Igor Belluschi1, Bruno Merlanti1, Benedetta De Chiara2, Marco Solcia3, Claudio F Russo1
Case summary
In June 2024, an 82-year-old lady affected by severe symptomatic aortic valve stenosis was admitted to our center to perform elective transcatheter aortic valve implantation (TAVI). Transthoracic echocardiography (TTE) showed a mean and peak gradient of 45 and 80 mmHg, respectively, as well as severe pulmonary hypertension.
At the age of twelve, the patient had undergone left subclavian flap aortoplasty for the treatment of aortic coarctation (CoA). CT-scan revealed a challenging scenario when planning aortic pathway: a 21 mm residual luminal coarctation was identified at the isthmus, revealing an 80-degree acute angle between descending aorta and aortic arch (Figure A; Supplemental Video). Subclavian accesses were not available due to previous surgical ligation (left) and extreme tortuosity with calcified plaques (right).
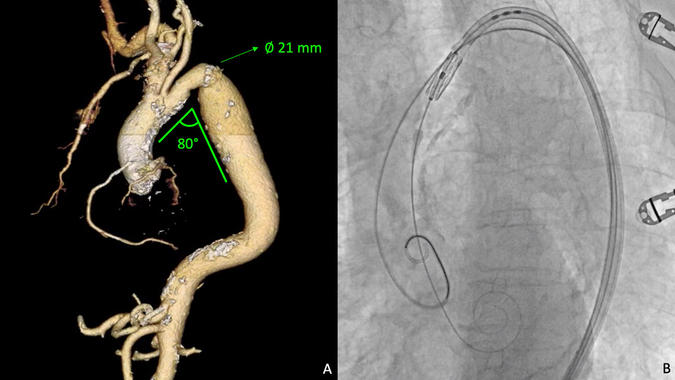
3D reconstruction CT-scan analysis shows aortic re-coarctation with a residual minimum diameter of 21 mm and a challenging acute angle of almost 80 degrees between descending aorta and aortic arch (A). Left subclavian artery is not visible due to previous surgical correction of CoA. A buddy-wire strategy by using an extra-stiff Lunderquist support together with a highly flexible Edwards Commander delivery system helped in crossing safely the residual stenosis (B).
Given advanced age and high surgical risk (EuroSCORE II 20.17 %), the Heart Team preferred a percutaneous approach by TAVI, despite the barriers in transcatheter heart valve (THV) delivery.
A 20-mm Sapien 3 Ultra prosthesis (Edwards Lifesciences, Irvine, CA, USA) was selected on data imaging. CT-scan analysis was performed, using AW VolumeShare (Version 4.5, GE HealthCare, Chicago, IL, USA). The procedure took place in hybrid OR under mild sedation. An interventional radiologist expert in endovascular treatment of thoracic aortic disease joined us.
After percutaneous preparation of both common femoral accesses with a 45 cm 6 Fr introducer on the left for pigtail angiography and a 14 Fr eSheat on the right for THV delivery, an extra-stiff 300 cm Lunderquist guidewire (Cook Medical, Bloomington, IN, USA) was first placed in ascending aorta through a pig-tail catheter as buddy-wire (Figure B; Supplemental Video). No gradient has been identified across the residual coarctation. Once the aortic valve has been crossed, an extra-small super-stiff pre-shaped Safari guidewire (Boston Scientific, Natick, MA, USA) was positioned in the left ventricle. Under rapid pacing (180 bpm), the Sapien 3 prosthesis was efficiently released with nominal filling volume. Post-procedural TTE showed no residual leaks and a transprosthetic gradient of 10 mmHg.
The procedural outcome of TAVI has been exponentially improving in the last decades due to several aspects: an outstanding technological growth, precise pre-procedural planning, and enhanced technical skills. However, some anatomical limitations remain unsolved.
Indeed, TAVI target population embraces a variety of atherosclerotic patients suffering of high-grade arterial tree disease, leading to complex scenarios when treating them by TAVI.
Focusing on THV delivery, not only the peripheral and the aortic annulus play essential roles - likewise airplane takeoff and landing - but also the “aortic itinerary”. In this case, we delivered the THV very gently through a residual aortic lumen restriction and unfavorable arch angle: the buddy-wire approach with a second extra-stiff guidewire, along with the possibility to control flexibility of the Edwards Commander delivery system, offered a very helpful support.
In 2021, a report of adult patients affected by congenital heart disease (ACHD) including n = 6/13 cases with history of surgery for CoA and who underwent TAVI showed no mortality or paravalvular leak > grade 2 at 30 days1. The risk of re-coarctation lesions during THV delivery should be relatively low since residual tissue appeared calcified after surgery and previous cases of post-TAVI aortic dissection were more commonly reported in the context of fragile and enlarged aortas2.
Nonetheless, the definition of “challenging aorta” includes a wide spectrum of aortic diseases: horizontal aortas3,4, vessel tortuosity, previous aortic dissection and the presence of dangerous atherosclerotic plaques or thrombi are only some of the main issues that every TAVI specialist should keep in mind when approaching the aortic pathway. Despite still debated, some of those scenarios may have a bad prognosis if not treated by expert hands.
To conclude, in the context of pushing TAVI through the boundaries of heavily arterial co-morbid patients, aortas previously treated for coarctation with adequate residual lumen appear to be crossable. However, accurate planning and advanced technical skills remain pivotal to optimize patients’ safety.
Supplementary material
References
- Moharem-Elgamal S, Yeong M, Veerappan S, Manghat N, Bedair R, Dorman S, et al. Feasibility and effectiveness of transcatheter aortic valve implantation in adults with congenital heart disease, International Journal of Cardiology Congenital Heart Disease. 2021; 3:100116;
- Fujita H, Ito T, Kikuchi S, Seo Y. Postprocedural ascending aortic dissection after transcatheter aortic valve implantation: a case report, European Heart Journal - Case Reports. 2023 Jan;7(1);
- Gallo F, Gallone G, Kim WK, Reifart J, Veulemans V, Zeus T, Toggweiler S, et al. Horizontal Aorta in Transcatheter Self-Expanding Valves: Insights From the HORSE International Multicentre Registry. Circ Cardiovasc Interv. 2021 Sep;14(9):e010641;
- Di Stefano D, Colombo A, Mangieri A, Gallone G, Tzanis G, Laricchia A, et al. Impact of horizontal aorta on procedural and clinical outcomes in second-generation transcatheter aortic valve implantation. EuroIntervention. 2019 Oct 4;15(9):e749-e756.
*Affiliations
- ASST Grande Ospedale Metropolitano Niguarda, Heart Transplant & Cardiac Surgery Unit, “De Gasperis” Cardio-Thoracic and Vascular Department, Milan – Italy
- ASST Grande Ospedale Metropolitano Niguarda, Echocardiography Unit, “De Gasperis” Cardio-Thoracic and Vascular Department, Milan – Italy
- ASST Grande Ospedale Metropolitano Niguarda, Interventional Radiology Unit, Milan – Italy
Conflicts of interest
The Authors have no conflict of interest to declare.





