TOP-CABG: the timing of platelet inhibition after coronary artery bypass grafting trial
Reported from ESC Congress 2025
Jonathan Curio provides his take on TOP-CABG, presented by Xin Yuan at ESC Congress 2025 in Madrid.
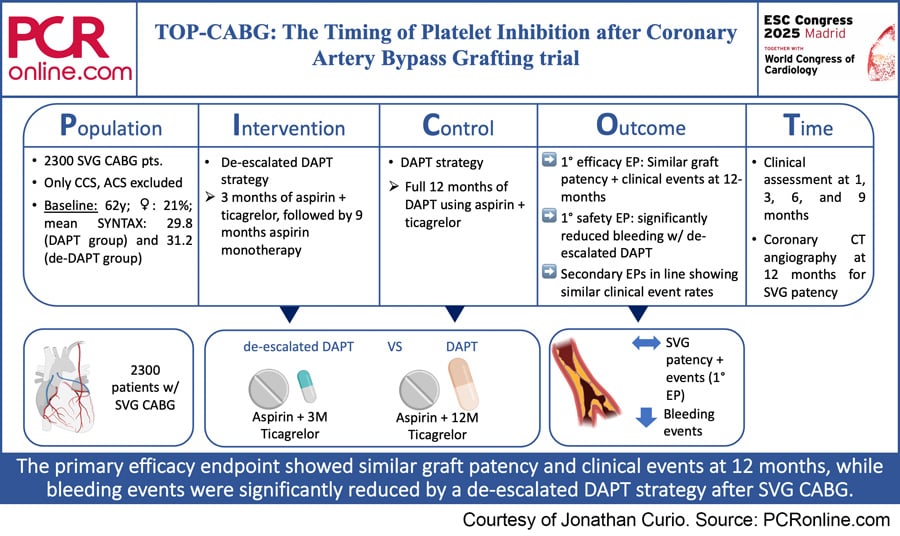
PICOT analysis of TOP-CABG
Courtesy of Jonathan Curio. Source: PCRonline.com
Why this study? - the rationale/objective
Occlusion of saphenous vein grafts (SVG) is one of the most feared complications after coronary artery bypass graft (CABG) surgery. Around 20 % of SVGs occlude during 1-year follow-up after CABG, resulting in recurrence of angina, potentially leading to events of myocardial infarction and, finally, impacting mortality.
Antiplatelet therapy following CABG using aspirin can be considered as standard of care, while a dual antiplatelet therapy (DAPT) strategy combining aspiring with a P2Y12 inhibitor might be of benefit in terms of improved graft patency and associated better clinical outcomes. Such potential benefits need to be balanced against a higher bleeding risk following a more aggressive antiplatelet strategy.
Apart from a straightforward DAPT regimen, a de-escalated DAPT (de-DAPT) strategy initially using aspirin combined with a more potent P2Y12 inhibitor in the first three months after CABG and then de-escalating to aspirin monotherapy for the following nine months can be considered to balance DAPT risks and benefits.
Against this background, the TOP-CABG (The Timing of Platelet Inhibition after Coronary Artery Bypass Grafting) trial aimed to compare a DAPT with a de-DAPT strategy following SVG CABG surgery.
How was it executed? – the methodology
The TOP-CABG trial was a multicentre, randomised, double-blind, parallel controlled trial, which was conducted in 13 centers across China. The trial included 2,300 patients receiving CABG with one or more SVGs. Eligible patients were randomised on post-operative day 5 to either a DAPT (ticagrelor + aspirin for 12 months) or a de-DAPT (ticagrelor + aspirin for 3 months, followed by 9 months of aspirin monotherapy) strategy. Patients were followed at 1, 3, 6, and 9 months via outpatient visit or telephone interview, and, after 12 months, patients additionally received a coronary computed tomography angiography (CCTA).
Endpoints of the trial included:
- Primary efficacy endpoint: SVG patency at 1-year follow-up as per CCTA or coronary angiography
- Primary safety endpoint: bleeding events (Bleeding Academic Research classification ≥ 2) within 1 year after CABG
- Secondary endpoints included general SVG failure (composite of SVG occlusion, revascularisation, myocardial infarction in the supplied territory, or sudden death); significant (≥ 70 %) SVG stenosis; and MACCE episodes within 1 year after CABG
What is the main result?
Mean age of the trial population was 61.6 years (DAPT group) and 61.3 years (de-DAPT group), 20.7 % (DAPT group) and 20.5 % (de-DAPT group) of the patients were female, mean SYNTAX score was 29.8 (DAPT group) and 31.2 (de-DAPT group), and the patients in both groups received a mean of 2.5 SVGs. SVG characteristics (type, location) were well balanced between both groups.
- According to the primary endpoint, SVG occlusion at 12 months was similar in both DAPT (11.2 %) and de-DAPT (10.8 %) groups, reaching statistical significance for non-inferiority (p = 0.008)
- According to the primary safety endpoint a de-DAPT strategy could significantly reduce clinically relevant bleeding at 1-year (8.3 % vs. 13.2 % in DAPT group; HR 0.62, p < 0.001)
- Secondary outcomes were in line, showing no significant differences for SVG failure or occurrence of MACCE between the two strategies
Critical reading and the relevance for clinical practice
According to the TOP-CABG trial, the largest dedicated trial on DAPT after CABG, a de-DAPT strategy could significantly reduce clinically relevant bleeding events while providing similar clinical outcomes as compared to a more intensive full 12-months DAPT strategy.
These results provide a useful and eagerly awaited guidance on the antiplatelet therapy provided after SVG CABG. Prior studies, testing aspirin + clopidogrel in not only chronic coronary syndrome (CCS) but also acute coronary syndrome (ACS) patients, could not show a benefit of a DAPT strategy as compared to aspirin monotherapy. Using the more potent P2Y12 inhibitor ticagrelor according to the presented data may be a more effective strategy – as tested in the trial, most ideally provided in a de-escalated fashion. However, one major limitation of the trial is that an aspirin monotherapy arm was not included and, thus, this important other strategy of current clinical practice was not assessed.
Another key difference, when comparing this trial to previous studies, is the exclusion of ACS patients. It is uncertain how these tested strategies would perform outside of a CCS population.
Lastly, the exact mechanisms of SVG failure (platelet activity &/or intima hyperplasia), as well as their timing (early/in first three months after surgery vs. during later follow-up), still are not fully understood and, thus, the question remains how different DAPT strategies may interact with these mechanisms. However, as of now, it seems a fair argument to de-escalate DAPT after 3 months, as this represents a timepoint know to likely correlate with activated platelet function returning to baseline levels and SVG stenosis & MACCE mainly occurring in the first 3 months post CABG.
In conclusion, the TOP-CABG trial provides evidence that a de-escalated DAPT strategy may provide similar clinical outcomes, while showing significantly reduced bleeding events in comparison to a full 12-months DAPT strategy.
How this strategy performs against aspirin monotherapy and whether such strategy should be indicated for all SVGs or rather for higher risk scenarios (e.g., major coronary vessels) in lower bleeding risk patients remains to be tested in future studies.




No comments yet!