Controlled ring expansion during aortic valve-in-valve: a proof-of-concept CT-scan analysis
Supported by the EuroIntervention Journal
Authors*
Giuseppe Bruschi1, Bruno Merlanti1, Benedetta De Chiara2, Igor Belluschi1, Claudio F Russo1
Case summary
In September 2020, a 63-year-old man affected by severe aortic valve stenosis and mitral valve regurgitation underwent elective surgical aortic valve replacement with 23-mm Inspiris Resilia and mitral annuloplasty with 28-mm Physio ring (Edwards Lifesciences, Irvine, CA, USA).
Two years after surgery, he developed chest pain and dyspnea. Transthoracic echocardiography revealed increased transaortic gradients (mean 48, peak 82 mmHg), without prosthesis regurgitation1. No valve thrombosis or vegetations on the bioprosthesis were reported.
Despite relatively low surgical risk (EuroSCORE II 1.83 %), the Heart Team opted for a percutaneous approach by valve-in-valve instead of surgical reintervention according to the patient’s preference for a bioprosthetic choice. A 26-mm Sapien 3 transcatheter heart valve (Edwards Lifesciences, Irvine, CA) was chosen for ViV implantation to enable future coronary access and improve downstream TAVI procedure, thanks to the expandable valve ring (VFit technology).
CT-scan analysis was performed using AW VolumeShare 4 (GE HealthCare, Chicago, IL, USA).
The procedure took place in hybrid OR under mild sedation. Under rapid pacing (200 bpm), the Sapien 3 prosthesis was released with nominal filling volume first and later with an additional of 1.5 ml. Thanks to balloon valvuloplasty, Inspiris ring was enlarged in a controlled manner from 23 mm size to 25 mm (Figure).
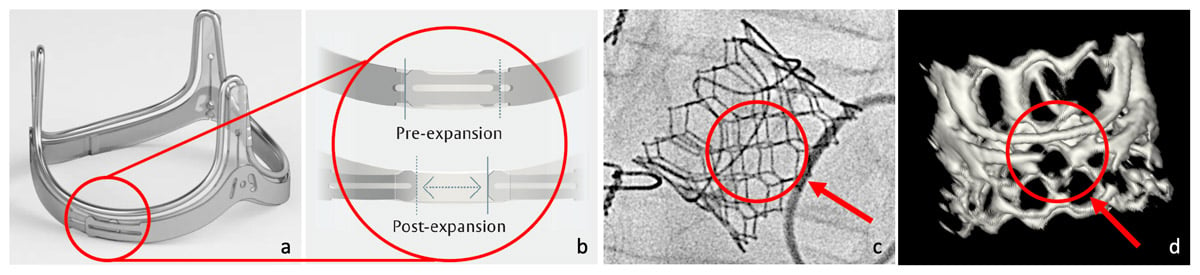
The Edwards Inspiris prosthesis stent (a). The expanding ring technology (b) seen both under fluoroscopy (c) and CT-scan (d) allows an increase at least in one size as reported by the manufacturer. In this case, it consisted in ~20% prosthesis area expansion from 399 mm2 to 494 mm2.
Post-procedural echocardiography showed no residual gradient or intra/para-valvular leaks. The reason for early Inspiris structural valve deterioration has not been identified in our case, however the patient was discharged asymptomatic 4 days later.
A few reports describing ViV in Inspiris prosthesis have been published2,3, but post-valvuloplasty imaging confirming diameter increase because of VFit mechanism was limited. The VFit technology concept consists of a controlled ring expansion of the Inspiris bioprosthesis, allowing at least one size increase of the THV, thus lowering residual gradients. Furthermore, the controlled expansion may prevent serious complications related to bioprosthetic ring fracture4. Another aspect offered by the manufacturer is the application of a prosthesis size marker on the stent pivots, detectable by fluoroscopy, which may help the cardiologist in a faster choice of the right device for ViV procedures, even when no patient history is available.
The advantages of choosing the Edwards Inspiris prothesis are not only related to VFit technology. In fact, this biological valve represents the latest manufacturer’s evolution of the previous Carpentier-Edwards Perimount and Magna Ease devices, thus reflecting their well-known good hemodynamic performances5.
Furthermore, thanks to the new Edwards Inspiris Resilia biological tissue and its glutaraldehyde stabilization, optimal prevention against calcium structural valve deterioration has been reported at bench testing, thus a promised very long durability is expected. The increase in effective orifice area provided by VFit mechanism could open the way to a long-awaited trend towards further valve-in-valve-in-valve procedures, especially when a big size of the Inspiris prosthesis is surgically implanted. In this manner, a lifespan biological prosthesis strategy - so required by young patients trying to avoid the anticoagulation burden - may be truly pursued.
Nevertheless, some limitations in the choice of this device should be addressed. Indeed, despite the interesting improvements offered by this bioprosthesis, we should remember that the 2021 ESC/EACTS guidelines on valve disease still recommend the choice of a bioprosthesis in patients aged > 65 years in the aortic position (class IIa, level C)6. Additionally, cases of valve thrombosis have already been reported in literature, even for the Inspiris device in specific context7, so caution when targeting the young population should be always remembered.
Moreover, during surgical aortic valve replacement, an annular decalcification process is performed after removing the stenotic native valve, but some atherosclerotic plaques in heavily calcified annuli may be left in site by the surgeon to avoid aortic wall lacerations. So, during procedural ViV planning, the profile of extremely fragile patients with weak and residual annular calcific components should be considered, despite thanks to the VFit technology the bioprosthesis stent fracture is avoided in favor of a more delicate stent and annular enlargement. In this setting, a gentle and slow balloon full inflation should be performed, thus dilating the Inspiris surgical stent in a controlled manner.
To conclude, the introduction of surgical aortic bioprosthesis with the benefit of expandable ring technology should definitively improve upcoming ViV procedures. However, further studies are necessary to confirm the hemodynamic and survival advantages of this technology.
Supplementary material
References
- Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, Bax JJ, Leipsic JA, Blanke P, Blackstone EH, Finn MT, Kapadia S, Linke A, Mack MJ, Makkar R, Mehran R, Popma JJ, Reardon M, Rodes-Cabau J, Van Mieghem NM, Webb JG, Cohen DJ, Leon MB. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J Am Coll Cardiol. 2021 Jun 1;77(21):2717-2746.
- Mehdiani A, Chekhoeva A, Klein K, Lichtenberg A. The first report of transcatheter aortic valve-in-valve implantation within the expandable Inspiris Resilia® bioprosthetic valve. Eur J Cardiothorac Surg. 2022 Jul 11;62(2):ezac394.
- Batlivala SP, Hagel JA, Hirsch R, Shahanavaz S. Transcatheter pulmonary valve-in-valve implantation within the expandable Inspiris Resilia® bioprosthetic valve. Catheter Cardiovasc Interv. 2022 Mar;99(4):1157-1160.
- Allen KB, Chhatriwalla AK, Saxon JT, Huded CP, Sathananthan J, Nguyen TC, Whisenant B, Webb JG. Bioprosthetic valve fracture: a practical guide. Ann Cardiothorac Surg. 2021 Sep;10(5):564-570.
- Porto A, Stolpe G, Badaoui R, Boudouresques V, Deutsch C, Amanatiou C, Riberi A, Gariboldi V, Collart F, Theron A. One-year clinical outcomes following Edwards INSPIRIS RESILIA aortic valve implantation in 487 young patients with severe aortic stenosis: a single-center experience. Front Cardiovasc Med. 2023 Aug 3;10:1196447.
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022 Feb 12;43(7):561-632.
- De Martino A, Falcetta G, Colli A. Early Valve Thrombosis in Surgical Aortic Bioprosthesis: Rare or Underestimated Event? J Am Coll Cardiol. 2020 Oct 13;76(15):1812.
*Affiliations
- ASST Grande Ospedale Metropolitano Niguarda, Heart Transplant & Cardiac Surgery Unit, “De Gasperis” Cardio-Thoracic and Vascular Department, Milan – Italy
- ASST Grande Ospedale Metropolitano Niguarda, Echocardiography Unit, “De Gasperis” Cardio-Thoracic and Vascular Department, Milan – Italy
Conflicts of interest
The Authors have no conflict of interest to declare.






No comments yet!