25 Jan 2021
Interventional management of an aortic root fistula after aortic-valve endocarditis
Supported by the EuroIntervention Journal
Authors
Christina Paitazoglou1, Ulrich Lange2, Jörn Sandstede3, Martin W. Bergmann1
Case summary
Left-sided endocarditis accounts for 85% of all endocarditis cases, with perivalvular extension more common in the aortic valve endocarditis.1 Perivalvular complications of infective endocarditis include abscess and fistulae; the frequency of fistula formation is rare (1.6-3.5%), with S. aureus being the most commonly associated organism (46%).2 Additionally, acquired aortocavital fistulas are an infrequent entity, which may occur late after an episode of endocarditis of the native aortic valve.3
In-hospital mortality of aortic root fistulas after endocarditis is high (41%) and they traditionally require surgical intervention.1,2 However, percutaneous catheter closure after successful valve endocarditis treatment is a novel method for selected cases.3 This is a patient with an aortic root fistula to the right heart after past infective aortic valve endocarditis. Interventional shunt occlusion was successfully performed using a transcatheter closure device.
A 44-year-old male presented with a new continuous 3/6 grade heart murmur and exertional dyspnoea six months after past aortic valve (blood culture negative) endocarditis. He had been treated with an antibiotic regiment according to the current ESC guidelines and underwent dental surgery to eliminate chronic periodontitis believed to be the potential source of bacteraemia.
Transthoracic echocardiography revealed a left-right shunt in the aortic root from the non-coronary sinus of Valsalva into the right atrium (RA). Further evaluation included computed tomography (CT) showing the fistula in the base of the non-coronary sinus of Valsalva (Figure 1A) and an aortic angiography with confirmation of the aortic shunt and passage of a right diagnostic catheter (JR4) into the RA and right ventricle (RV) with consecutive pressure drop from 114/75 to 46/8 mmHg (Figure 1B). Left-right shunt volume calculation showed a shunt fraction of less than 20% (Qp:Qs < 1.2).
Transoesophageal echocardiography (TEE) ruled out ongoing vegetation’s (Figure 1C). Acute ongoing infective endocarditis was ruled out according to clinical judgement (no fever and signs of infection in any laboratory measurements on CRP and leucocyte count) and the modified Duke criteria for infectious endocarditis.
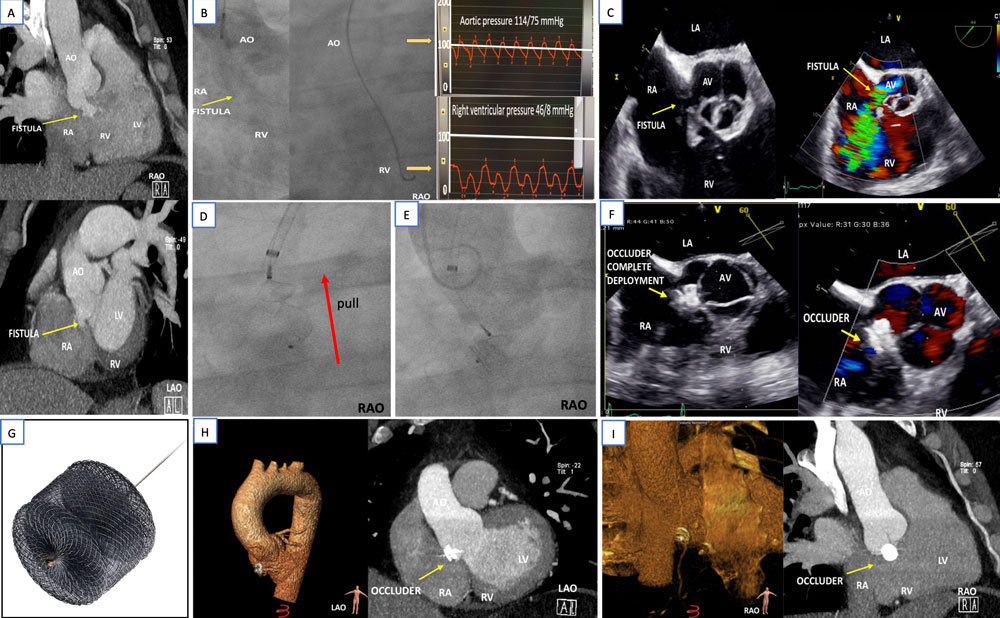
Figure 1: Interventional approach of an aortic fistula after endocarditis
A) Computed tomography (RAO- and LAO-images) before occlusion show the left-right shunt in base of the non-coronary sinus of Valsalva. B) Angiography of the aortic root confirmed the left-right shunt and the pressure changes after catheter passage through the fistula. C) Transoesophageal images of the short axis depict the fistula in the aortic root. D-E) Fluoroscopic and F) echocardiographic images of the deployment of the distal (right-atrial) disc, the central waist and proximal (aortic) disc (red arrow shows the pull manoeuvre to prove stability before deployment) of the G) Amplatzer Vascular Plug device II (Abbott) without remaining shunt or aortic valve obstruction. H-I) Computed tomography with three-dimensional reconstruction after two years demonstrates the interventional aortic root fistula occlusion.
Untreated aortic fistulas may cause significant morbidity and lead to increased mortality due to progressive tissue destruction and once recognised, fistula closure appears to be superior to conservative management.4 Even though the shunt volume was small, the young patient was believed to be in danger of chronic heart failure or pulmonary hypertension, therefore interventional fistula occlusion was offered.
Images of the procedure are depicted in Figures 1D-F and supplementary material (moving images 1-5).
Following arterial puncture of the right femoral artery, the fistula was negotiated by a JR4 diagnostic catheter which allowed to place a Safari wire into the RV guided by fluoroscopy and TEE. A 6F delivery system was placed into the RV. After pre-interventional CT and TEE sizing, an Amplatzer Vascular Plug II 12mm device (Figure 1G) was securely loaded and advanced through the delivery system into the RV. Firstly, the distal disc was deployed (on the right heart side) and then the central waist and the proximal disc in the non-coronary sinus of Valsalva under constant pull. As expected from pre-interventional imaging, the device completely occluded the shunt to the RA (Figure 1F).
A two-year clinical and CT follow-up in our outpatient clinic was performed. Patient presented without heart failure or infect symptoms. CT showed the device to be in situ with total shunt occlusion (Figure 1H-I).
Conflict of interest statement
No conflicts of interest to declare.
Affiliations
- Interventional Cardiology, Cardiologicum Hamburg, Germany
- Asklepios Klinik Wandsbek, Hamburg, Germany
- Radiologische Allianz, Hamburg, Germany
References
- Habib G, Lancellotti P and Iung B. 2015 ESC Guidelines on the management of infective endocarditis: a big step forward for an old disease. Heart. 2016;102:992-4.
- Anguera I, Miro JM, Vilacosta I, Almirante B, Anguita M, Munoz P, San Roman JA, de Alarcon A, Ripoll T, Navas E, Gonzalez-Juanatey C, Cabell CH, Sarria C, Garcia-Bolao I, Farinas MC, Leta R, Rufi G, Miralles F, Pare C, Evangelista A, Fowler VG, Jr., Mestres CA, de Lazzari E, Guma JR and Aorto-cavitary Fistula in Endocarditis Working G. Aorto-cavitary fistulous tract formation in infective endocarditis: clinical and echocardiographic features of 76 cases and risk factors for mortality. Eur Heart J. 2005;26:288-97.
- Said SA and Mariani MA. Acquired aortocameral fistula occurring late after infective endocarditis: An emblematic case and review of 38 reported cases. World J Cardiol. 2016;8:488-95.
- Foster TJ, Amin AH, Busu T, Patel K, Farjo P, Hallak AA, Ali N and Alkhouli M. Aorto-cardiac fistula etiology, presentation, and management: A systematic review. Heart Lung. 2020;49:317-323.




2 comments
is there a risk for vascular plug to cause occlussion of valve leaflet
Thanks for the question, I see it only now. There may certainly be a risk, that's why we took a lot of time intraprocedural to confirm by TOE that the plug is not affecting leaflet movement and is not changing position despite rigorous tug tests. The patient is still fine and we feel this procedure was the best option compared to conservative treatment or surgery.