MASTER-DAPT: “the MASTER of high-bleeding risk trials”
Reported from the European Society of Cardiology ESC Congress 2021
Marco Valgimigli (Lugano, Switzerland) presented the results of the MASTER DAPT trial on Saturday, August 28, in a Hot Line session of the ESC Congress 2021. Luis G. Ortega-Paz provides his PICOT & SWOT analysis of this study.
High-bleeding risk (HBR) patients who require coronary stenting have been a subject of intense research in recent years.
Marco Valgimigli presented the results of the MASTER DAPT trial (Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Prolonged DAPT Regimen) on behalf of the investigators during ESC 2021.
The complete rationale and design of the trial was previously published in 20191. Besides, in #EuroPCR 2021, a comprehensive analysis of short DAPT in high bleeding risk patients, including the MASTER DAPT trial design, was done.
Now, we will perform a PICOT and SWOT analysis for this coverage.
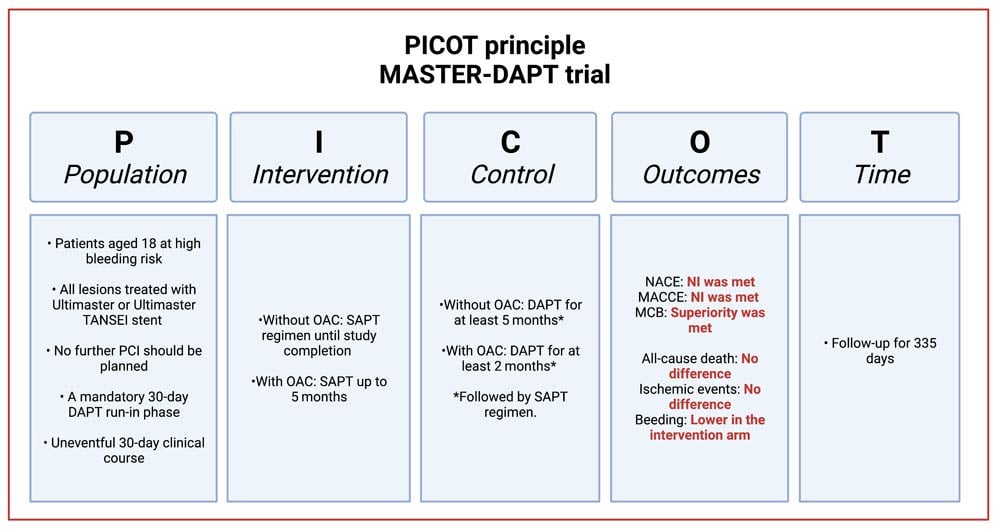
Examination of the study methodology and results according to the PICOT principle.
Population:
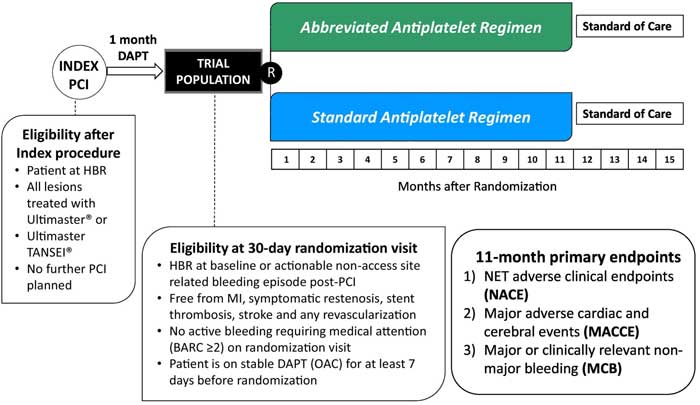
- Patients aged 18 at high bleeding risk.
- All lesions treated with Ultimaster or Ultimaster TANSEI stent.
- No further PCI should be planned.
- A mandatory 30-day dual antiplatelet therapy (DAPT) run-in phase.
- Uneventful 30-day clinical course.
Figure 1. Study design and key features1.
Source: American Heart Journal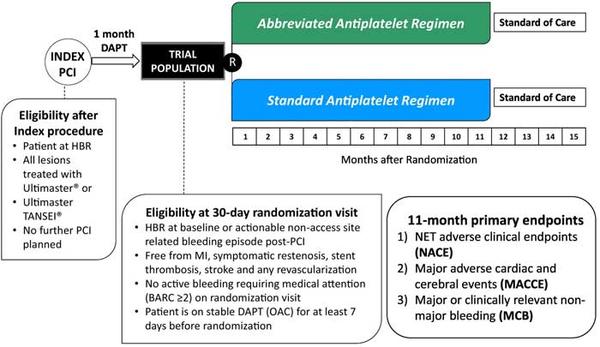
Intervention
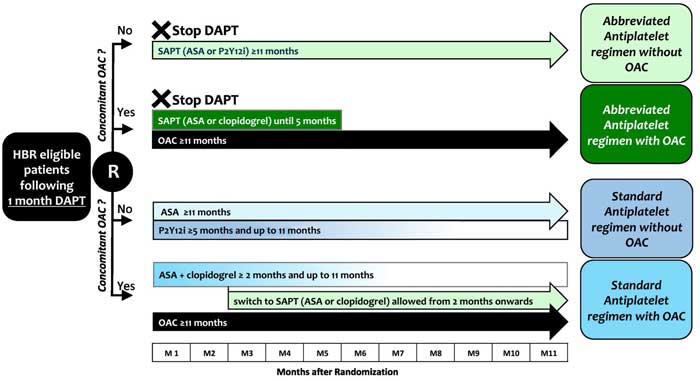
- Single antiplatelet regimen until study completion or up to 5 months in patients with clinically indicated oral anticoagulation (OAC).
Control
- DAPT for at least 5 months in patients without or for at least 2 months in patients with concomitant indication to OAC, followed by a single antiplatelet regimen.
Figure 2. Treatment in the experimental and control arm1.
Source: American Heart Journal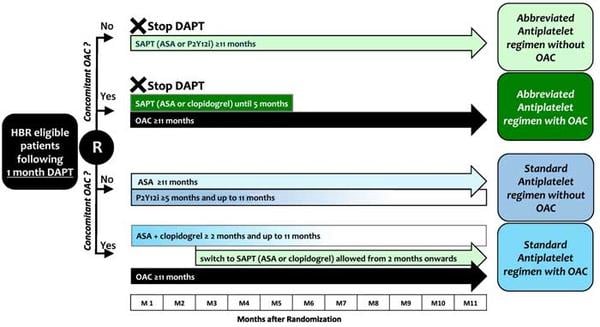
Outcomes2
- A total of 4,434 patients were included.
- There were 3 co-primary endpoints tested in a hierarchical order (definitions according to the protocol 1):
- Net adverse clinical endpoints (NACE): Non-inferiority was met between intervention and control (7.5 % vs. 7.7 %; P < 0.001). Per-protocol population.
- Major adverse cardiac and cerebral events (MACCE): Non-inferiority was met between intervention and control (6.1 % vs. 5.9 %; P < 0.001). Per-protocol population.
- Major or clinically relevant non-major bleeding (MCB): Superiority was met between intervention and control (6.5 % vs. 9.4 %; P < 0.001). Intention-to treat population.
- Key secondary endpoints included (Per-protocol):
- All-cause death and cardiovascular (CV) death:
- All-cause death: not statistically different (intervention: 3.3 % vs. control 3.6 %).
- CV death: not statistically different (intervention: 1.7 % vs. control 2.0 %).
- Ischemic events:
- Myocardial infarction (MI): not statistically different (intervention: 2.7 % vs. control 2.1 %).
- Stroke/TIA: not statistically different (intervention: 0.7 % vs. control 1.4 %).
- Definite/probable device thrombosis: not statistically different (intervention: 0.6 % vs. control 0.4 %).
- Bleeding events (Bleeding Academic Research Consortium3):
- BARC 1-5: Lower in the intervention arm (intervention: 8.7% vs. control 13.2%).
Time
- Follow-up for 335 days.
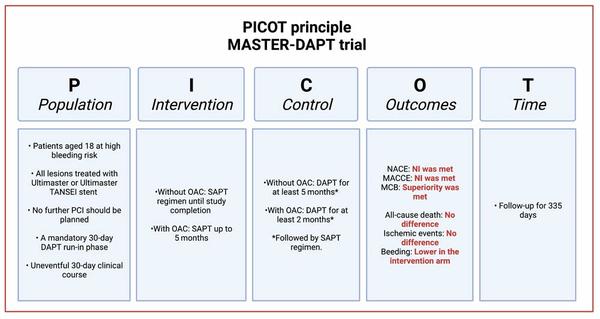
Analysis according to the PICOT principle - courtesy of Luis Ortega Paz
- PCI: percutaneous cardiac intervention;
- DAPT: dual antiplatelet therapy;
- SAPT: single antiplatelet therapy;
- OAC: oral anticoagulation;
- NACE: Net adverse clinical endpoints;
- MACCE: Major adverse cardiac and cerebral events;
- MCB: Major or clinically relevant non-major bleeding.
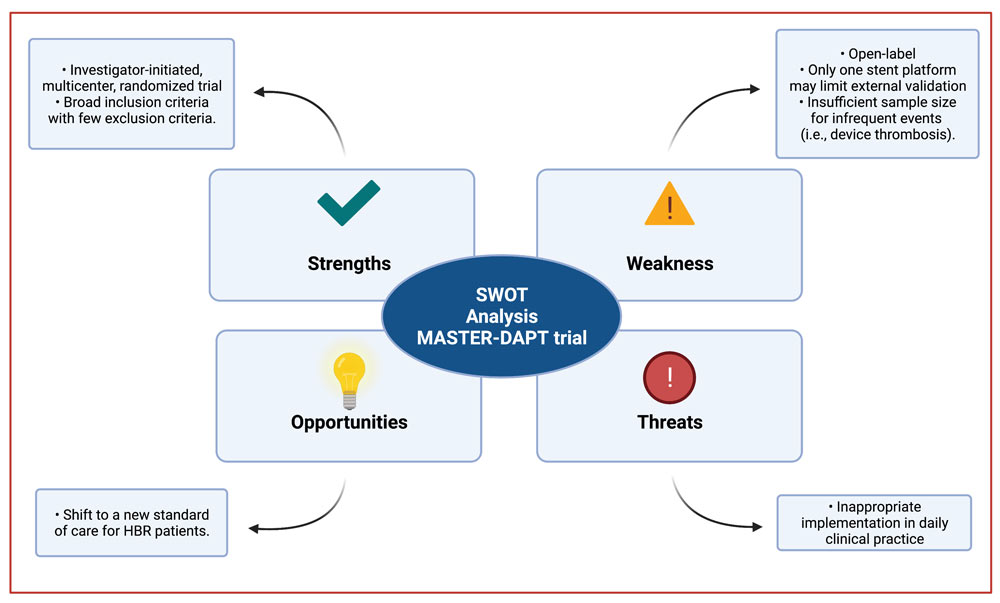
Strengths – Weaknesses – Opportunities – Threats (SWOT) analysis
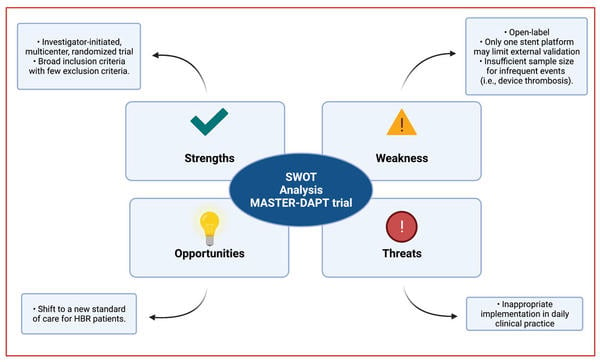
SWOT Analysis - courtesy of Luis Ortega Paz
Related interview
References
- Frigoli E, Smits P, Vranckx P, Ozaki Y, Tijssen J, Juni P, Morice MC, Onuma Y, Windecker S, Frenk A, Spaulding C, Chevalier B, Barbato E, Tonino P, Hildick-Smith D, Roffi M, Kornowski R, Schultz C, Lesiak M, Iniguez A, Colombo A, Alasnag M, Mullasari A, James S, Stankovic G, Ong PJL, Rodriguez AE, Mahfoud F, Bartunek J, Moschovitis A, Laanmets P, Leonardi S, Heg D, Sunnaker M and Valgimigli M. Design and rationale of the Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Standard DAPT Regimen (MASTER DAPT) Study. Am Heart J. 2019;209:97-105.
- Valgimigli M, Frigoli E, Heg D, Tijssen J, Jüni P, Vranckx P, Ozaki Y, Morice M-C, Chevalier B, Onuma Y, Windecker S, Tonino PAL, Roffi M, Lesiak M, Mahfoud F, Bartunek J, Hildick-Smith D, Colombo A, Stanković G, Iñiguez A, Schultz C, Kornowski R, Ong PJL, Alasnag M, Rodriguez AE, Moschovitis A, Laanmets P, Donahue M, Leonardi S and Smits PC. Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk. New England Journal of Medicine. 2021.
- Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG and White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-47.




No comments yet!