OPTION – Indobufen or aspirin on top of clopidogrel after coronary drug-eluting stent implantation
Reported from AHA 2022
Ali Nazmi Calik provides his take on OPTION, which was presented during AHA 2022 in Chicago.

Courtesy of Ali Nazmi Calik
Why this study? The rationale/objective
Dual antiplatelet therapy (DAPT), including a P2Y12 inhibitor and aspirin, is the mainstay of treatment after percutaneous coronary intervention (PCI).
Allergic reactions, intolerance, and hypersensitivity along with resistance are the drawbacks of aspirin therapy that led to the investigation of alternative pharmacological agents1,2,3.
Indobufen, a reversible cyclooxygenase-1 (COX-1) inhibitor, reduces platelet thromboxane (TX) production with a negligible impact on prostacyclin (PGI2) production. Thus, unfavorable effects of aspirin, such as gastrointestinal intolerance, are less frequently observed with indobufen, while maintaining similar platelet inhibition4.
Despite being used as an alternative regimen in patients unable to receive aspirin after stent deployment, data regarding indobufen therapy on top of a P2Y12 inhibitor is scarce5,6.
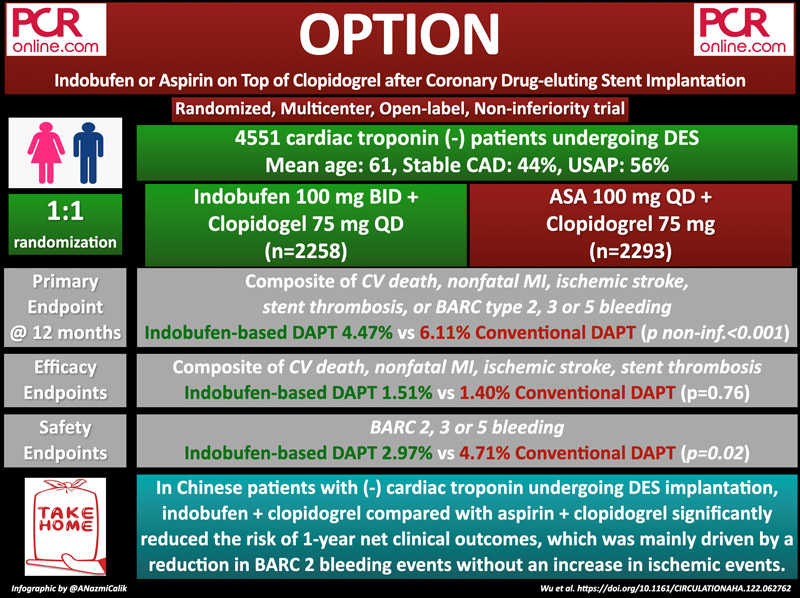
The OPTION trial was designed to compare the 1-year clinical efficacy and safety of indobufen-based DAPT (indobufen plus clopidogrel) or conventional DAPT (aspirin plus clopidogrel) in patients with negative cardiac troponin undergoing coronary drug-eluting stent (DES) implantation.
How was it executed? – the methodology
The OPTION trial is a randomized, open-labeled, prospective, non-inferiority, multicenter study conducted in 103 cardiovascular centers in China. Patients aged between 18 to 75 years old, diagnosed with coronary artery disease with symptoms of angina pectoris or documented ischemia, with negative cardiac troponin (T or I) on admission, treated with at least one DES implanted in the coronary lesion in the past 24 hours, were enrolled in this study.
Exclusion criteria included complicated PCI procedure, elevated cardiac troponin (T or I) on admission, MI within a year, elective surgical procedure planned within 12 months, known allergy or intolerance to aspirin, clopidogrel or nonsteroidal anti-inflammatory drugs (NSAIDs), history of cerebral hemorrhage, history of stroke in past six months, active bleeding, known relevant hematological deviations, active cancer, severe hepatic or renal insufficiency known active peptic ulcer, concomitant use of oral anticoagulants, clinically important thrombocytopenia (i.e. platelet < 100*109/L) or anemia (i.e. hemoglobin < 90g/L), pregnancy or lactation.
In the indobufen-based DAPT group, patients who were not on aspirin were given indobufen 200 mg immediately after randomization. In patients who were pretreated with a cumulative dose of aspirin less than 300 mg, a dose of indobufen 100 mg was continued after randomization.
In the aspirin-based DAPT group, patients who were aspirin naive and those who were pretreated with a cumulative dose of aspirin less than 300 mg, a dose of aspirin 100 to 300 mg was given immediately after randomization.
Both groups received DAPT (indobufen 100 mg bid + clopidogrel 75 mg qd or aspirin 100 mg qd + clopidogrel 75 mg qd) for 12 months.
The primary endpoint was a composite of cardiovascular (CV) death, nonfatal MI, ischemic stroke, definite or probable stent thrombosis (ST), or Bleeding Academic Research Consortium (BARC) criteria type 2, 3, or 5 bleeding at 1 year.
The secondary efficacy outcome was a composite of CV death, nonfatal MI, ischemic stroke, and definite or probable ST. The secondary safety outcome included BARC criteria type 2, 3, or 5 bleeding.
What is the main result?
A total of 4,551 patients were randomly assigned to receive either indobufen-based DAPT (n = 2258) or conventional DAPT (n = 2293). The mean age was 61.1 and 2,968 (65.2 %) patients were men. 43.6 % of the patients were diagnosed with stable coronary artery disease (CAD) whereas 2,568 (56.4 %) suffered unstable angina.
At 1 year, the primary endpoint occurred in 101 (4.47 %) patients in the indobufen-based DAPT group and 140 (6.11 %) patients in the conventional DAPT group (absolute difference -1.63 %, 95 % CI -2.93 % to -0.33 %, pnoninferiority < 0.001). Kaplan–Meier event curves showed a significant reduction in the risk of the primary endpoint in the indobufen group (HR 0.73, 95 % CI 0.56 to 0.94, p = 0.015).
The secondary efficacy endpoints, which is a composite of CV death, nonfatal MI, ischemic stroke, and definite or probable ST, occurred in 34 (1.51 %) patients in the indobufen-based DAPT group and 32 (1.40 %) patients in the conventional DAPT group (HR 1.08, 95 % CI 0.67 to 1.75, p = 0.76). BARC 2, 3, or 5 bleeding events were significantly lower in the indobufen group (2.97 % vs 4.71 %, HR 0.63, 95 % CI 0.46 to 0.85, p = 0.002), a finding mainly driven by the difference between BARC 2 bleeding events (1.68 % vs 3.49 %, HR 0.48, 95 % CI 0.33 to 0.70, p < 0.001).
Critical reading and the relevance for clinical practice
In Chinese patients with negative cardiac troponin undergoing DES implantation, indobufen plus clopidogrel DAPT for 12 months is non-inferior to aspirin plus clopidogrel with regard to the primary endpoint. Moreover, the occurrence of net adverse clinical events was significantly lower in the indobufen group (HR 0.73, 95 % CI 0.56 to 0.94, p = 0.01), which was mainly driven by a significantly reduced risk of BARC 2 bleeding events without an increase in ischemic events.
A few points should be considered while implementing the results of this study into daily practice. First, despite being the largest randomized comparison of indobufen with aspirin, open-label design and lower-than-expected event rates (8 % vs 6.1 % in the aspirin group) mitigate the impact of the study.
Second, the advantage of the indobufen was confined to less minor bleeding events rather than an improvement in ischemic endpoints.
Third, 12-month DAPT regimen is longer than the currently recommended DAPT duration following elective PCI. The clinical benefit of indobufen begins around 6 weeks, when de-escalation of DAPT by removing aspirin is widely practiced. This may limit the application of the study results to daily practice.
In conclusion, indobufen, which requires high patient adherence owing to its twice-daily regimen, is an alternative to aspirin in biomarker-negative patients undergoing PCI and treated with clopidogrel, particularly in cases of aspirin hypersensivity, intolerance, or resistance.
References
- Evidence Review Committee M, Bittl JA, Baber U, Bradley SM and Wijeysundera DN. Duration of Dual Antiplatelet Therapy: A Systematic Review for the 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2016;134:e156-78.
- Tournoij E, Peters RJ, Langenberg M, Kanhai KJ and Moll FL. The prevalence of intolerance for low-dose acetylsalicylacid in the secondary prevention of atherothrombosis. Eur J Vasc Endovasc Surg. 2009;37:597-603.
- Bhatt DL, Grosser T, Dong JF, Logan D, Jeske W, Angiolillo DJ, Frelinger AL, 3rd, Lei L, Liang J, Moore JE, Cryer B and Marathi U. Enteric Coating and Aspirin Nonresponsiveness in Patients With Type 2 Diabetes Mellitus. J Am Coll Cardiol. 2017;69:603-612.
- Rebuzzi AG, Natale A, Bianchi C, Mariello F, Coppola E and Ciabattoni G. Effects of indobufen on platelet thromboxane B2 production in patients with myocardial infarction. Eur J Clin Pharmacol. 1990;39:99-100.
- Latib A, Ielasi A, Ferri L, Chieffo A, Godino C, Carlino M, Montorfano M and Colombo A. Aspirin intolerance and the need for dual antiplatelet therapy after stent implantation: a proposed alternative regimen. Int J Cardiol. 2013;165:444-7.
- Barilla F, Pulcinelli FM, Mangieri E, Torromeo C, Tanzilli G, Dominici T, Pellicano M, Paravati V, Acconcia MC and Gaudio C. Clopidogrel plus indobufen in acute coronary syndrome patients with hypersensitivity to aspirin undergoing percutaneous coronary intervention. Platelets. 2013;24:183-8.




No comments yet!