The PRE18FFIR trial: Coronary Plaque Activity to Predict Recurrent Events
Reported from ESC Congress 2022
Alex Sticchi provides his take on the PRE18FFIR trial: Prediction of recurrent events with 18F-Sodium Fluoride to identify ruptured and high-risk coronary artery plaques in patients with myocardial infarction.
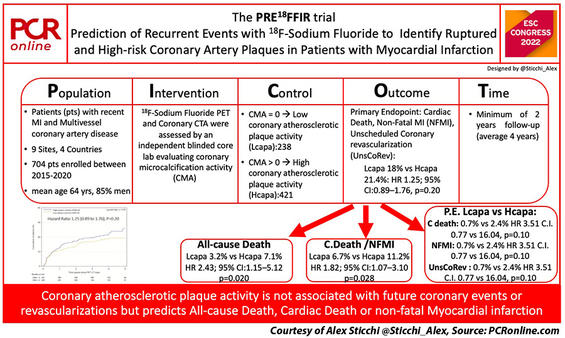
PICOT analysis of PRE18FFIR
Courtesy of Alex Sticchi @Sticchi_Alex, Source: PCRonline.com
The prediction of acute coronary syndromes and atherosclerotic complications remains one of the big questions in cardiovascular pathophysiology understanding.
Acute coronary syndromes are based on the idea of non-obstructive vulnerable plaques, and the identification of the characteristics of instability always represents a hot topic in the cardiology field.
Microcalcification is the most significant characteristic of inflammation predicting plaque rupture and correlating with histological evidence. The 18F-Sodium Fluoride-CT is able to detect early microcalcification and other minor plaque anatomical features of instability.
The PRE18FFIR Trial moved from the result of the post-hoc observational study of Fletcher et al.[1] that reported the association of high coronary 18F-Sodium Fluoride activity with myocardial infarction (HR: 4.8 [95% CI: 1.9-12.2]; P=0.00095) in stable coronary disease. So, this study investigated the role of plaque activity through microcalcification identification in predicting the risk of recurrent events and future cardiac death in high-risk patients.
The PRE18FFIR Trial is an international multi-center (9 sites in 4 countries) prospective longitudinal cohort study. It enrolled 704 patients between 2015 and 2020 with recent heart attacks and multivessel coronary artery disease after a screening of about two thousand patients. At study completion, follow-up was available for 98.2% of participants.
In the study, the investigators compared the outcomes of the Low versus the High coronary atherosclerotic plaque activity group, identified by the 8F-sodium fluoride PET and CTCA scans.
The Original Primary Endpoint was a composite of cardiac death or non-fatal myocardial infarction (MI). However, a correction was needed due to a low rate of events at the mid-point check (about 10% vs 20-30% initially estimated). Therefore, the Trial Steering Committee recommended the inclusion of unscheduled coronary revascularization into the combined primary endpoint, considering the relationship between plaque expansion and coronary activity.
The Primary Endpoint, a composite of cardiac death, non-fatal MI or unscheduled coronary revascularization, occurred in 20% of all study population and in 18% vs 21.4% of the low and high coronary atherosclerotic plaque activity groups, respectively (see PICOT image above). This result leads to a non-significant difference between the two groups with an HR of 1.25 (95% confidence interval [CI] 0.89 – 1.76), p=0.20.
No significant differences occurred in the single Primary Endpoint components.
An interesting result is a statistically significant difference in all-cause death (HR 2.43; 95% CI 1.15 – 5.12, p=0.020) and in the composite of cardiac death or non-fatal MI (HR 1.82, 95% CI1.07 – 3.10, p=0.028). However, no significant differences occurred in the unscheduled coronary revascularization (HR 0.98, 0.64 – 1.49, p=0.91).
Looking at the Original Primary Endpoint adding the adjustment for the contemporary GRACE risk score and the severity of obstructive coronary artery disease, a trend of risk occurred for the composite of cardiac death or non-fatal MI (HR 1.69, 95% CI 0.98 – 2.91, p=0.058), and a no statistically significant interaction for the all-cause death.
In terms of safety, the 18F-Sodium Fluoride PET-CT showed a favourable well-tolerated profile, with a low number of adverse events (0.0028%).
In summary, the PRE18FFIR Trial represents the first prospective international multi-center study using 18F-Sodium Fluoride PET-CT to evaluate the prognostic utility of plaque activity in high-risk patients. The study missed the predefined Primary Endpoint. The microcalcifications as plaque activity markers did not predict all coronary events and unscheduled revascularization. However, plaque activity showed to predict cardiac death or non-fatal MI and all-cause death. The 18F-Sodium Fluoride PET-CT seems to be safe, but it is a high-cost exam with inter-vendor biases on measurements.
Further studies using this technology and on this exciting topic will give us more information in the future.
References:
- Fletcher AJ, Tew YY, Tzolos E, Joshi SS, Kaczynski J, Nash J, Debono S, Lembo M, Kwiecinski J, Bing R, Syed MBJ, Doris MK, van Beek EJR, Moss AJ, Jenkins WS, Walker NL, Joshi N V., Pawade TA, Adamson PD, Whiteley WN, Wardlaw JM, Slomka PJ, Williams MC, Newby DE, Dweck MR. Thoracic Aortic 18F-Sodium Fluoride Activity and Ischemic Stroke in Patients With Established Cardiovascular Disease. Cardiovasc Imaging. 2022;15:1274–88.




No comments yet!