Primary Outcomes of a Pivotal Multicenter Randomized Trial Comparing the AGENT Paclitaxel-Coated Ballon with Conventional Ballon Angioplasty for In-Stent Restenosis (AGENT IDE)
Reported from TCT 2023
Mirvat Alasnag provides her take on the AGENT IDE trial which was presented by Robert W. Yeh at TCT Congress 2023. This pivotal randomized trial compares the safety and efficacy of the AGENT DCB to conventional balloon angioplasty in patients with ISR. It is the first randomized trial conducted in the United States reaffirming the efficacy and safety of DCB in patients with coronary ISR.
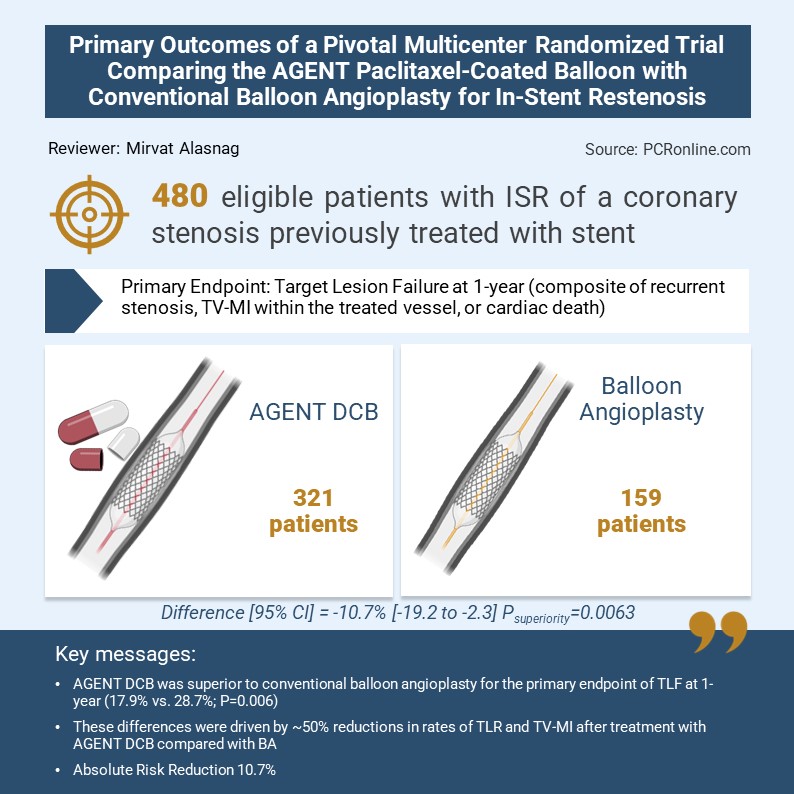
The design was pragmatic, namely, a prospective, randomized, multicenter, superiority trial across 40 US sites (N=480). The key Inclusion Criteria: Patients with ISR of a lesion previously treated with bare metal stent or drug eluting stent; lesion length 2.0 - ≤ 4.0 mm, and % diameter stenosis 50 – 100% in symptomatic individuals. A recent ST elevation myocardial infarction, bifurcation, Left Main disease, grafts, and thrombus in target vessel were the primary exclusion criteria. Patients were randomized 2:1 after successful pre-dilation of target lesion to DCB (321) vs conventional (159) balloon angioplasty.

The primary endpoint was Target Lesion Failure (TLF) at 1-year (composite of target lesion revascularization (TLR), target vessel-myocardial infarction (TV-MI), or cardiac death). Clinical follow-up consisted of in-hospital, 30 days, 6 months, 1-year and annually between 2 and 5 year intervals.
The first randomized trial conducted in the United States reaffirming the efficacy and safety of DCB in patients with coronary ISR
Based on an adaptive trial design, the final sample size was increased to 600 patients given the low event rate. The primary endpoint analysis was conducted on the first 480 patients which was evidently powered for events and presented at TCT LBCT session. The mean age was 67 years with 26% women. Diabetics constituted 51% and prior MI occurred in 48% of those enrolled. In addition, approximately 77% had multivessel disease. The 37% had an acute coronary syndrome (Non-ST elevation ACS) and over 50% had stable coronary artery disease. 48% had lesions 10-20 mm distributed across all three epicardial coronary arteries. Multiple stent layers were noted in 43% of the cohort making this an overall high-risk population. Technical and clinical success in the DCB arm exceeded 90%. Approximately 70% of those enrolled underwent intracoronary imaging with 50% using intravascular ultrasound. TLF at one year occurred in 17.9% of the AGENT arm and 28.7% of the angioplasty arm with a confidence interval [95% CI] = -10.7% [-19.2 to -2.3] and Psuperiority = 0.0063. The absolute risk reduction was 10.7% and an NNT of 10. This was driven by reducing the TV-MI (AGENT 6.4% vs. BA 12.3% HR 0.51 (95% CI 0.27 to 0.95) P=0.03) and TLR (AGENT 12.4% vs. BA 24.0% HR 0.49 (95% CI 0.31 to 0.78) P=0.002) by 50%. The rate of stent thrombosis was AGENT 0.0% vs. BA 3.9% P=0.001. The study was powered for other clinical endpoints. The results were consistent across all subgroups including age, sex, diabetes and layers of stents.
AGENT IDE is the first randomized trial conducted in the United States reaffirming the efficacy and safety of DCB in patients with coronary ISR which has been common practice outside the USA. The remaining question is whether the control arm should have been another drug eluting stent rather than a conventional balloon angioplasty. Given that over 43% had multiple stent layers, additional metallic stent layers is generally avoided. It would be interesting to study the role of DCB in other scenarios such as bifurcations with a provisional strategy and treatment of small diffuse distal disease.




No comments yet!