5-year outcomes after transcatheter or surgical aortic valve replacement in low-risk patients with aortic stenosis (Evolut Low Risk)
Reported from ACC.25
Yohei Ohno provides his takeaways from the results of the Evolut Low Risk trial which was presented by Michael J. Reardon during the ACC.25 in Chicago.
Why this study – the rationale/objective?
Transcatheter aortic valve implantation (TAVI) is currently the predominant intervention for the treatment of symptomatic severe aortic stenosis (AS) in the United States. However, there is limited long-term data in younger patients with longer life expectancy using supra-annular self-expandable valves.
The objective of Evolut low risk trial was to evaluate the safety and effectiveness of TAVI with Evolut as compared with surgical aortic valve replacement (SAVR) in patients with low surgical risk, and 5-year results were presented in a Late-Breaking Clinical Trial at ACC Congress 2025 with simultaneous publication in the JACC.
How was it executed?
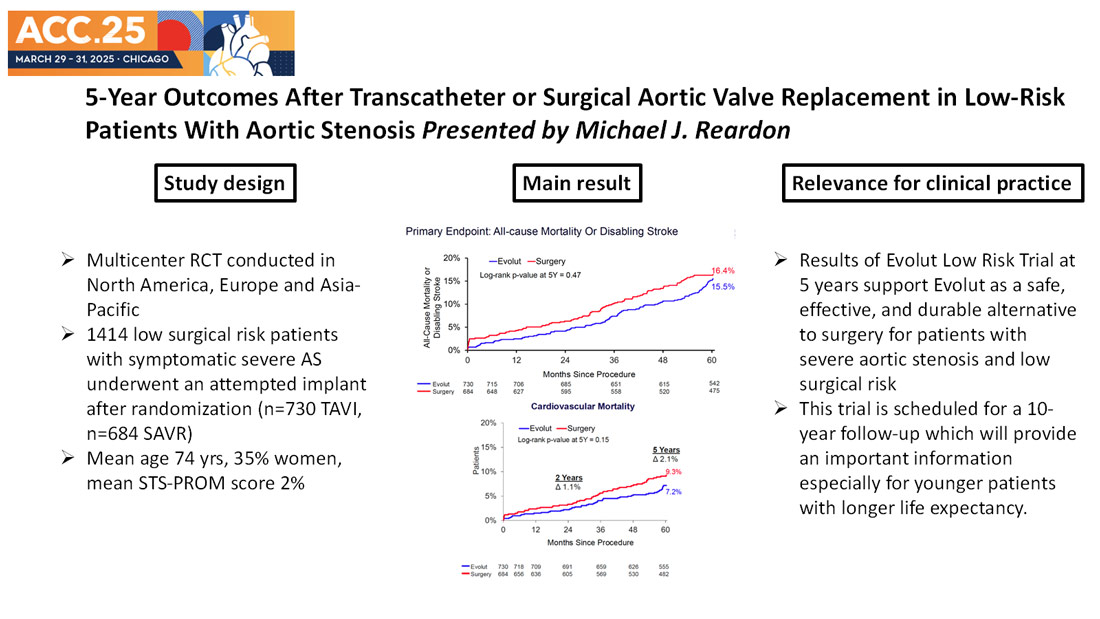
A total of 1,414 patients underwent an attempted intervention after randomization (n=730 TAVI, n=684 SAVR). At 5-year follow-up, approximately 90% of the patients were evaluable status (n=671 TAVI, n=598 SAVR).
The primary endpoint was a composite of all-cause mortality or disabling stroke at 2 years. Clinical endpoints reported in this analysis include 5-year rates of all-cause mortality or disabling stroke as well as valve performance as determined using Doppler echocardiographic assessment, paravalvular regurgitation (PVL) at 30 days and 5 years, quality of life as assessed using Kansas City Cardiomyopathy Questionnaire (KCCQ), and NYHA functional class. The mean age of patients was 74 years; 35% were women, mean STS-PROM score was 2%.
What is the main result?
Excerpt from Michael J. Reardon's presentation at ACC.25
- At 5 years the Kaplan-Meier (KM) estimate for the primary endpoint of all-cause mortality or disabling stroke was 15.5% for the TAVI group and 16.4% for the SAVR group (P = 0.47). The breakdowns of the primary endpoint were comparable between the two groups. Cardiovascular mortality was 7.2% in the TAVI group and 9.3% in the SAVR group (P = 0.15).
- Superior hemodynamic valve performance was demonstrated at all follow-up points up to 5 years. At 5 years, the mean aortic valve gradient was significantly lower after TAVI than SAVR (10.7 mmHg vs 12.8 mmHg; difference: -2.1; 95% CI: -3.0 to -1.2; P < 0.001) and mean effective orifice area was significantly larger, 2.1 cm2 TAVI vs 1.9 cm2 SAVR (difference: 0.2; 95% CI: 0.1-0.3; P < 0.001).
- Aortic valve reintervention occurred in 21 patients (3.3%) after TAVI and 14 patients (2.5%) after SAVR by 5 years (difference: 0.8%; 95% CI: -1.2% to 2.9%; P = 0.44).
- A sustained improvement in quality of life was observed in both treatment arms with mean Kansas City Cardiomyopathy Questionnaire summary score of 88.3 ± 15.8 in TAVI and 88.5 ± 15.8 in SAVR.
Critical reading and the relevance for clinical practice
The Evolut low risk trial is the first RCT to directly compare TAVI using Evolut vs. SAVR in patients with low surgical risk. It is an important study to demonstrate TAVI using Evolut as a safe, effective, and durable alternative to SAVR for patients with severe aortic stenosis and low surgical risk. The following points are the ones which I thought important and relevant for our daily clinical practice.
- Some may have concerns about the KM curve of primary endpoint which TAVI arm gets close to SAVR arm between 4 and 5 years. This was predominantly due to increase in non-cardiovascular mortality during this study period. As shown in the Figure, consistent lower number of cardiovascular mortality is shown in TAVI compared to SAVR, although not statistically significant.
- Incidence of both clinical and subclinical valve thrombosis was comparable between the two groups which was not demonstrated in the previously reported low risk trial. It is known that valve design leads to these differences which is supported by fluid mechanics study (Midha PA et al. Circulation 2017). This is considered to be one of the reasons why this supra-annular platform Evolut provides the longest durability data to date.
- Incidence of reintervention and its outcome were similar between the groups, with surgical approach being the majority type of intervention in both groups. Whether some of the TAVI reintervention could have been performed as re-do TAVI using leaflet modification techniques needs further investigation.
- There were treatment variations in both arms: Older generation Evolut devices were used in TAVI arm (CoreValve 3.6%, Evolut R 74.1%, Evolut PRO 22.3%), bioprosthesis used in SAVR arm was Edwards 57%, Abbott 19%, Medtronic 11%, Livanova 14% which also includes types of bioprosthesis no longer used.
When performing TAVI as a treatment for younger patients with longer life expectancy, durability of the transcatheter heart valve and its potential re-do TAVI feasibility (repeatability) should always be considered. With this reassuring 5-year data and considering the fact that new Evolut platform, i.e. Evolut FX+ is likely to achieve commissural and coronary alignment in majority of the patients, Evolut would be a reasonable choice in this cohort. However, it is still a 5-year result and we definitely need a longer term follow-up which this trial is scheduled for a 10-year follow-up.






No comments yet!