Routine cerebral embolic protection in transcatheter aortic valve implantation: the British Heart Foundation (BHF) PROTECT-TAVI trial
Reported from ACC.25
Aaysha Cader and Ali Nazmi Calik provide their take on the BHF PROTECT-TAVI trial, presented by Rajesh Kharbanda at ACC.25 in Chicago.
Designed by Aaysha Cader & Ali Nazmi Calik. Source: PCRonline
Why this study – the rationale/objective?
Transcatheter Aortic Valve Implantation (TAVI/TAVR) is a standard treatment for severe aortic stenosis. Despite increasing safety, stroke (which can be caused by embolism, hemorrhage, or cardiovascular collapse with cerebral hypoperfusion), remains an unpredictable complication in patients undergoing TAVI. Cerebral embolic protection (CEP) devices are designed to capture debris, and thereby reduce embolic stroke, and the Sentinel CEP (Boston Scientific) is the only CEP approved for use in the USA and Europe.
However, clinical studies to date, including the large global PROTECTED TAVR trial1, have been inconclusive in this regard. In PROTECTED TAVR, which enrolled 3,000 patients, there was no difference in the incidence of 72-hour stroke between those who received a cerebral embolic protection (CEP) device and those who did not. However, disabling strokes occurred less frequently in patients who received a CEP.
How was it executed – the methodology?
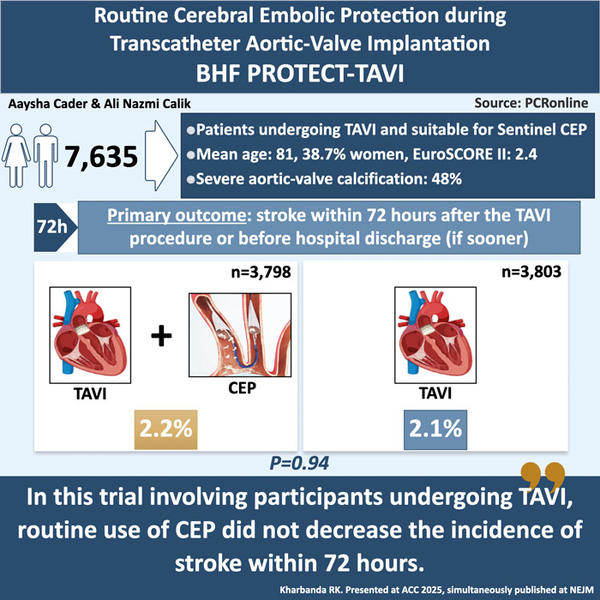
The PROTECT TAVI trial was a prospective, open-label, multicenter, randomised, controlled trial with blinded adjudication of the outcomes. A total of 7,635 patients were enrolled across 33 centres in the United Kingdom, which was representative of 29.6 % of all TAVI procedures undertaken across the National Health Service (NHS) and participating sites. Patients were randomised 1:1 to undergo TAVI with a CEP device (CEP group) or TAVI without a CEP device (control group).
The primary outcome was stroke within 72 hours after TAVI or before discharge from the hospital (if discharge occurred sooner). Importantly, the definition of stroke was clinical, and not defined by imaging alone: stroke was defined as neurological deficit that occurred after randomisation, persisted for > 24hours (or resulted in death within 24 hours) and included ischemic and haemorrhagic events. Stroke events were adjudicated by an independent clinical events committee that were blinded to treatment allocation.
Formal interim analyses were planned at 50 % and 70 % enrollment to assess efficacy and futility. Enrolment was discontinued prematurely as pre-specified futility criterion had been met. Pre-specified subgroup analyses were undertaken for the primary endpoint.
What is the main result?
A total of 7,635 patients were recruited: 3,815 participants assigned to CEP group, and 3,820 to the control group. The mean (± SD) age was 81.2 ± 6.5 years, 38.7 % were women and baseline characteristics of patients were representative of a UK TAVI population. Mean aortic valve gradient was 43 mmHg.
A primary-outcome event occurred in 81 of 3,795 participants (2.1 %) in the CEP group, and in 82 of 3,799 participants (2.2 %) in the control group (difference, −0.02 percentage points; 95 % confidence interval, –0.68 to 0.63; P = 0.94). No differences in outcomes were seen in subgroup analyses.
There were also no differences in other secondary endpoints including all-cause death (0.8 % in the CEP group, and in 0.7 % in the control group arms), and cognitive outcomes, or severe stroke which occurred in 0.5 % each in the CEP and control groups (difference, 0.0 percentage points; 95 % CI, –0.3 to 0.3). Disabling stroke within 6 to 8 weeks of TAVI occurred in 47 participants (1.2 %) in the CEP group, and in 53 (1.4 %) in the control group. Most strokes occurred within 24 hours after the TAVI procedure.
Critical reading and the relevance for clinical practice
The BHF PROTECT-TAVI trial aimed to determine whether routine use of the Sentinel CEP device during TAVI could effectively reduce periprocedural stroke rates. Despite enrolling a large cohort of 7,635 patients, the trial demonstrated no significant reduction in stroke. Although a lower incidence of overall stroke was noted in PROTECT TAVI, this outcome aligns closely with the PROTECTED TAVR trial1, which also failed to show a meaningful clinical benefit of CEPDs in preventing disabling strokes.
Unlike the PROTECTED TAVR trial, the definition of adherence required both device filters to be fully deployed throughout the procedure. Additionally, BHF PROTECT-TAVI trial had less restrictive eligibility criteria, allowing a larger proportion of patients undergoing TAVI to be enrolled, including those with complex access or aortic arch anatomy who would have been excluded from PROTECTED TAVR. These factors may account for some of the differences in device deployment rates between the two trials.
A notable finding from the BHF PROTECT-TAVI trial was the absence of any signal suggesting benefit in subgroup analyses. High-risk patients, including those with severe aortic calcification or previous cerebrovascular events, did not experience a reduction in stroke rates, contradicting assumptions that CEPs might selectively benefit these groups. The lack of efficacy in all patient subgroups raises questions about the utility of CEPs in TAVI procedures and suggests a need for further investigation into patient selection criteria.
Prior studies, including the MISTRAL-C trial2, CLEAN-TAVI trial3, and SENTINEL trial4, have shown that the SENTINEL device, designed with dual filters to capture embolic debris during TAVI, reduces the volume of cerebral lesions detected by MRI. However, these subclinical findings have not consistently translated into meaningful reductions in clinical stroke rates or cognitive decline. The current design, which partially protects the brain’s vasculature, may contribute to the limited clinical impact observed. Given the limitations of the current CEPs, future iterations may focus on more comprehensive cerebral protection, improved device deployment, and broader embolic capture capabilities.
Looking ahead, an upcoming patient-level meta-analysis combining data from the BHF PROTECT-TAVI and PROTECTED TAVR may provide a more nuanced understanding of CEPD efficacy. This analysis will focus on identifying subgroups that might benefit from protection and refining clinical recommendations for CEPD use in TAVI.
The trial has certain limitations. Although consecutive enrollment of participants was encouraged, the trial was conducted during the COVID-19 pandemic, which significantly impacted clinical research activity in the United Kingdom. Furthermore, despite efforts to recruit a diverse population, the majority of participants were White, with underrepresentation of minority racial or ethnic groups (Asian, Black, mixed, or other), limiting the generalisability of the findings.
In conclusion, while the BHF PROTECT-TAVI trial did not demonstrate a benefit from routine CEPD use, the evolving landscape of cerebral protection technology and ongoing analyses may yet define a role for selective use in high-risk patients.
References
- Kapadia SR, et al. Cerebral Embolic Protection during Transcatheter Aortic-Valve Replacement. N Engl J Med. 2022 Oct 6;387(14):1253-1263
- Van Mieghem NM, et al. Filter-based cerebral embolic protection with transcatheter aortic valve implantation: the randomised MISTRAL-C trial. EuroIntervention. 2016 Jul 20;12(4):499-507.
- Haussig S, et al. Effect of a Cerebral Protection Device on Brain Lesions Following Transcatheter Aortic Valve Implantation in Patients With Severe Aortic Stenosis: The CLEAN-TAVI Randomized Clinical Trial. JAMA. 2016 Aug 9;316(6):592-601.
- Kapadia SR, et al. Protection Against Cerebral Embolism During Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2017 Jan 31;69(4):367-377
Authors







No comments yet!