Treatment effects of Interleukin-6 receptor antibodies for modulating the systemic inflammatory response after out-of-hospital cardiac arrest: the IMICA Trial
Selected in AHA Journal - Circulation by P. Hill , K. De Silva
The IMICA trial explored the effect of a single 1-hour infusion of Tocilizumab, initiated in patients as early as feasible in their hospital admission, post-cardiac arrest, and sought to investigate whether this would reduce the systemic inflammatory response.
References
Authors
Martin Abild Stengaard Meyer, Sebastian Wiberg, Johannes Grand, Anna Sina Pettersson Meyer, Laust Emil Roelsgaard Obling, Martin Frydland, Jakob Hartvig Thomsen, Jakob Josiassen, Jacob Eifer MØller, Jesper Kjaergaard, Christian Hassager
Reference
10.1161/CIRCULATIONAHA.120.053318
Published
March,22 2021
Link
Read the abstractReviewers
Our Comment
Why this study? – the rationale/objective
The first landmark multicentre report of outcomes of patients post-cardiac arrest was published in 1953, declaring an in-hospital mortality rate of 50 %1.
The cause and treatments have evolved dramatically over the past 60 years but the outcomes remain poor.
Between 1995-2005, in the United Kingdom, 71.4 % of 8,987 patients admitted to the Intensive Care Unit (ICU), following an out-of-hospital cardiac arrest, died before being discharged from the hospital2.
Post cardiac arrest syndrome (PCAS) is a critical disorder comprising post-cardiac-arrest brain injury, myocardial dysfunction and a systemic ischaemia/reperfusion response3.
The systemic response is a major contributor to the post-cardiac arrest state and IL-6 levels correlate with its severity4.
Interleukin-6 (IL-6) is a pro-inflammatory cytokine which has historically been a target for treatment in auto-immune conditions but more recently in the treatment of COVID-195.
Tocilizumab is a recombinant humanised monoclonal antibody which targets both the soluble and membrane-bound forms of the IL-6 receptor6.
Gupta et al saw a dramatic reduction of mortality in those critically ill patients in ICU, when Tocilizumab was administered within 2 days of their ICU admission7.
However, this remains unproven in the context of cardiac arrest secondary to acute coronary syndromes, with the IMICA (IL-6 inhibition for Modulating Inflammation after Cardiac Arrest) trial being the first undertaken in this setting.
The IMICA trial explored the effect of a single 1-hour infusion of Tocilizumab, initiated in patients as early as feasible in their hospital admission post-cardiac arrest, thought to be cardiac in aetiology. They sought to investigate whether this would reduce the systemic inflammatory response by measuring CRP (as a surrogate for IL-6), along with leukocyte levels, and hence diminish end-organ injury in PCAS.
How was it executed? - the methodology
This study was an investigator-initiated, randomized, placebo-controlled, double-blinded, single-centre, clinical phase II trial, with patients recruited from the Department of Cardiology, Rigshospitalet, Copenhagen, Denmark.
Patients were allocated 1:1 to receive either Tocilizumab or placebo.
The inclusion criteria were: OHCA of a presumed cardiac cause, > 18 years of age, GCS < 9, and sustained return of spontaneous circulation > 20 minutes.
Exclusion criteria included: > 240 minutes from return of spontaneous circulation until randomization, unwitnessed asystole, suspected or confirmed intracranial bleeding or stroke, pregnancy, temperature on admission < 30 °C, persistent cardiogenic shock (defined as systolic blood pressure < 90 mm Hg despite relevant interventions) that was not reversed within the inclusion window, any known disease making 180-day survival unlikely, known limitations in therapy, known prearrest Cerebral Performance Category of 3 to 4, known allergies to IL-6RA, known infections, and known hepatic cirrhosis.
The primary objective was to assess the response of C Reactive Protein (CRP) from the administration of Tocilizumab. A reduction in CRP was felt to reflect the biological effect of circulating IL-6. n = 117 consecutive patients were screened, with n = 80 meeting inclusion criteria. n = 39 were assigned to tocilizumab and n = 41 assigned to placebo (Figure 1).
Characteristics along with adverse/side effects within both cohorts were well balanced.
The secondary clinical endpoints included ICU length of stay, SOFA (Sequential Organ Failure Assessment) score (total and cardiovascular on days 1-3), haemodynamic data in the form of cardiac output and pulmonary capillary wedge pressure from right heart catheterisation, survival, safety and neurological outcomes (cerebral performance category and ranking scale at 30, 90 and 180 days).
Source: Circulation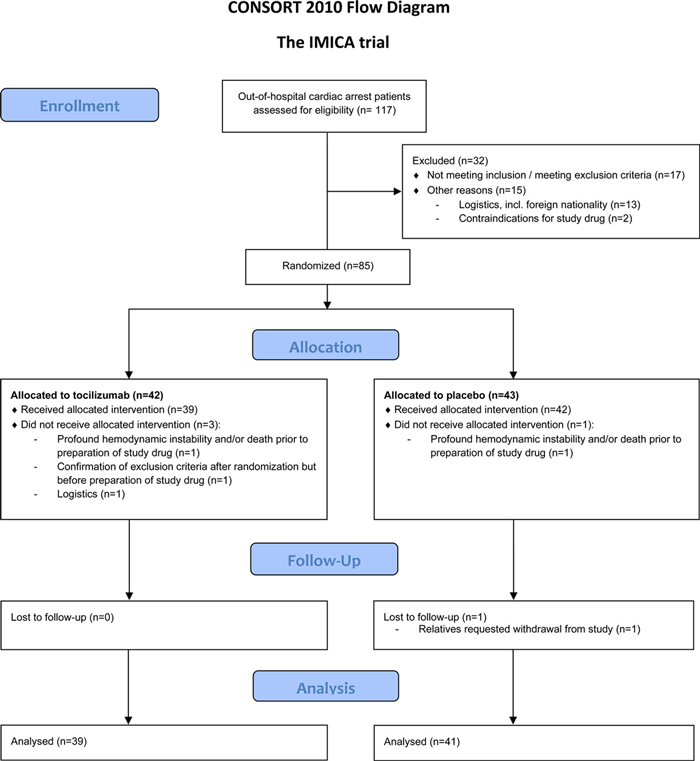
Consort flow diagram illustrating the trial design8
What is the main result?
Primary endpoint of a reduction in CRP was achieved (by 96 % after 72 hours) in the tocilizumab arm.
However, no significant difference was observed between the two groups in time spent in ICU, ventilator days or SOFA scores on day 1 and day 3.
Nonetheless, there was a marginally lower SOFA cardiovascular score noted in the tocilizumab cohort on day 3 along with a trend for fewer patients to experiences a temperature > 38 degrees celcius.
At 30-days, mortality rates were similar in the Tocilizumab (35.9 %) and placebo (34.1 %) arms (p = 1.00).
Moreover, the Neuronal specific enolase (NSE), modified Rankin and cerebral performance did not differ between the 2 groups at 48 and 72 hours post-OHCA.
From a cardiac-specific perspective, there was a substantial decrease in the secondary ‘cardioprotection’ objectives measured in this trial.
At 6-hours, TnT (33 %, p = 0.0017) and CKMB 36 %, p = < 0.0001) were both reduced in the Tocilizumab arm, suggesting a reduction in myocardial injury in those that were administered Tocilizumab. N-terminal pro-B-type natriuretic peptide (NT-proBNP), a marker of myocardial stress, was also reduced by 65 % at 48 hours in the same group (p = 0.0002).
Critical reading and the relevance for clinical practice
Out-of-hospital cardiac arrest continues to have exorbitantly high mortality despite contemporary cardiac and intensive care.
This is the first randomized trial of the use of a novel anti-inflammatory drug, Tocilizumab in this setting.
The trial investigators suggest that treatment with Tocilizumab results in a significant reduction in the inflammatory response and myocardial injury in comatose patients following OHCA from a presumed cardiac cause, evidenced by the reduction in CRP, TnT, and CK-MB.
From a cardiac standpoint, the studied group was heterogeneous, with only 33.3 % of the Tocilizumab group and 58.5 % of the placebo group having documented ST-elevation on their ECG, suggesting an acute myocardial infarction as the underlying cause of the cardiac arrest.
The selection criteria used to capture “probable cardiac cause” in OHCA and the explanation of clinical markers which led to urgent/emergent invasive coronary angiography remains unclear as are the numbers that underwent urgent/emergent revascularization with PCI.
This study adds to the literature that the systemic inflammatory and immunological process that occurs following a cardiac arrest is likely to be an important factor in patient recovery and outcomes, and many provide a unique biologic focus for treatment of such patients in the future. This is particularly pertinent in view of the poor outcomes that persist despite current gold-standard therapies in both, intensive and cardiac, care.
Whilst there was a signal of improvement in cardiac biomarkers (in TnT and CKMB), this did not translate to an improvement in clinical markers of recovery (e.g SOFA total score) or mortality.
However, as this was a descriptive study of a novel biologic agent and not powered for clinically meaningful endpoints, it remains hypothesis-generating only.
Therefore, the successful reduction in biomarkers observed in this study and its clinical impact needs to be assessed prospectively, in an adequately powered randomized trial, to determine whether there is potential clinical benefit in Tocilizumab therapy in the cardiac arrest setting.
References
- Stephenson HE, Reid LC, Hinton JW. SOME COMMON DENOMINATORS IN 1200 CASES OF CARDIAC ARREST: Ann Surg. 1953 May;137(5):731–44.
- Nolan JP, Laver SR, Welch CA, Harrison DA, Gupta V, Rowan K. Outcome following admission to UK intensive care units after cardiac arrest: a secondary analysis of the ICNARC Case Mix Programme Database*: ICNARC Case Mix Programme Database. Anaesthesia. 2007 Nov 5;62(12):1207–16.
- Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Böttiger BW, et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. Resuscitation. 2008 Dec;79(3):350–79.
- Bro-Jeppesen J, Kjaergaard J, Wanscher M, Nielsen N, Friberg H, Bjerre M, et al. Systemic Inflammatory Response and Potential Prognostic Implications After Out-of-Hospital Cardiac Arrest: A Substudy of the Target Temperature Management Trial*. Crit Care Med. 2015 Jun;43(6):1223–32.
- Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19 – Preliminary Report [Internet]. Infectious Diseases (except HIV/AIDS); 2020 Jun [cited 2021 Aug 17]. Available from: http://medrxiv.org/lookup/doi/10.1101/2020.06.22.20137273
- Kavanaugh A. Interleukin-6 inhibitors in the treatment of rheumatoid arthritis. Ther Clin Risk Manag. 2008 Aug;Volume 4:767–75.
- Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, et al. Association Between Early Treatment With Tocilizumab and Mortality Among Critically Ill Patients With COVID-19. JAMA Intern Med. 2021 Jan 1;181(1):41.
- Meyer MAS, Wiberg S, Grand J, Meyer ASP, Obling LER, Frydland M, et al. Treatment Effects of Interleukin-6 Receptor Antibodies for Modulating the Systemic Inflammatory Response After Out-of-Hospital Cardiac Arrest (The IMICA Trial): A Double-Blinded, Placebo-Controlled, Single-Center, Randomized, Clinical Trial. Circulation. 2021 May 11;143(19):1841–51.





No comments yet!