30 Nov 2022
Distal vs conventional radial access for coronary angiography and/or intervention: a meta-analysis of randomized trials
Selected in JACC: Cardiovascular Interventions by A. Cader , A. Félix De Oliveira
The present systematic review and meta-analysis by Ferrante et al aimed to provide a comprehensive quantitative analysis of the effects of DRA vs TRA, by pooling data from randomised controlled trials (RCT) comparing these two access sites when used for coronary angiography and/or percutaneous coronary intervention (PCI)5.
References
Authors
Giuseppe Ferrante, Francesco Condello, Sunil V. Rao, Matteo Maurina, Sanjit Jolly, Giulio G. Stefanini, Bernhard Reimers, Gianluigi Condorelli, Thierry Lefèvre, Samir B. Pancholy, Olivier Bertrand, and Marco Valgimigli
Reference
J Am Coll Cardiol Intv. 2022 Nov, 15 (22) 2297–2311
Published
November 2022
Link
Read the abstract
Reviewers
Our Comment
Why this study – the rationale/objective?
Despite its many advantages, conventional transradial access (TRA) is associated with radial artery occlusion (RAO), with pooled estimates of ~7.7 % at 24 hours1. An occluded radial artery limits its future use for percutaneous procedures, arterial conduits for coronary artery bypass grafting (CABG), and arteriovenous fistulae (AVF) for patients requiring haemodialysis2.
The distal radial access (DRA) has emerged as an alternative access site for percutaneous procedures, with potential for reduced forearm RAO, as haemostatic compression is performed at the anatomical snuffbox instead of the distal forearm3,4.
The present systematic review and meta-analysis by Ferrante et al aimed to provide a comprehensive quantitative analysis of the effects of DRA vs TRA, by pooling data from randomised controlled trials (RCT) comparing these two access sites when used for coronary angiography and/or percutaneous coronary intervention (PCI)5.
How was it executed? - the methodology
Eligibility criteria & databases searched:
An electronic search of MEDLINE, Embase, Cochrane Central Register of Clinical Trials and ClinicalTrials.gov databases was undertaken. RCTs comparing DRA vs conventional TRA for coronary angiography and/or PCI, reporting predefined clinical outcomes were included, with no language, publication date, or publication status restrictions.
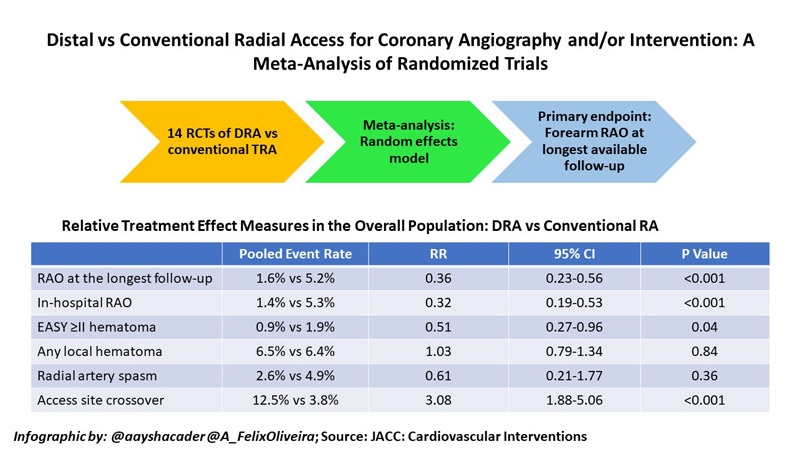
The primary endpoint of the meta-analysis was the rate of forearm RAO at the longest available follow-up. In-hospital RAO was assessed as a supportive secondary endpoint. Other secondary endpoints included local haematoma (defined as EASY or modified EASY ≥ II), radial spasm, time to successful radial artery puncture, time for sheath insertion, number of radial puncture attempts, fluoroscopy time, contrast volume and access site crossover5.
Statistical analysis:
Dichotomous outcomes were reported as risk ratios (RR) with 95 % confidence intervals. Heterogeneity between studies was evaluated using the Cochran’s Q chi-square test for between-studies variance Tau2 and using the I2 test to evaluate inconsistency6. A random-effects model was used for the analysis owing to the presence of high heterogeneity between studies. A meta-regression analysis was undertaken to adjust the study endpoints for co-variates. Also, sensitivity analyses were performed by including only studies published as full papers, excluding small studies, and by the leave-one-out method.
What is the main result?
From among 8,616 records identified, 6,290 de-duplicated records were screened, and a total of 14 RCTs were selected for the analysis, including 6,208 patients. Nine studies were full-paper publications, while 5 were in abstract form.
Radial artery occlusion:
RAO rates were reported in 13 out of 14 studies. The primary endpoint of RAO rates at longest follow-up was reported in 12 studies, all of which had a component of RAO detection by duplex ultrasonography (DUS). The study by Lucreziotti et al. was excluded from this analysis, presumably because RAO was only detected by manual palpation7. Moderate heterogeneity was found for RAO outcomes (RAO at the longest follow-up I2 = 28.3; in-hospital RAO I2 = 29.0).
Pooled event rates showed 1.6 % vs 5.2 % RAO at the longest follow-up for DRA vs TRA respectively, which ranged from 1 to 60 days. DRA use was associated with a 64 % reduction in forearm RAO, as compared with conventional TRA (RR: 0.36; 95 % CI: 0.23 to 0.56; P < 0.001). Similarly, 68 % reduced in-hospital RAO was seen for DRA (1.4 %) vs TRA (5.3 %; RR: 0.32; 95 % CI: 0.19 to 0.53; P < 0.001). On pre-specified meta-regression analysis, increasing age with the TRA group was associated with a reduced effect of DRA on the risk of RAO at the longest follow-up. Notably, however, no other variables (including female sex, smoking, diabetes, ACS presentation, PCI) emerged as treatment modifiers.
Secondary outcomes:
DRA also had 49,% reduced risk of EASY ≥ II hematoma (RR: 0.51; 95 % CI: 0.27 to 0.96; P = 0.04). However, there were no significant differences between DRA vs TRA in the risk of local hematoma (RR: 1.03; 95 % CI: 0.79 to 1.34; P = 0.84), radial spasm (RR: 0.61; 95 % CI:0.21 to 1.77; P = 0.36), or hemostasis time (standardised mean difference [SMD]: -2.32; 95 % CI: -5.43 to 0.78; P = 0.14).
Secondary endpoints related to obtaining access:
Perhaps reflecting anatomical location and learning curves8, DRA compared with conventional TRA was associated with a significantly higher time for radial artery puncture (SMD: 3.56; 95 % CI: 0.96 to 6.16; P < 0.001), sheath insertion (SMD: 0.37; 95 % CI: 0.16 to 0.58; P < 0.01), a higher number of puncture attempts SMD: 0.59, 95 % CI: 0.48 to 0.69; P < 0.001), and a higher risk of access site crossover (RR: 3.08; 95 % CI: 1.88 to 5.06; P < 0.001).
Source: JACC: Cardiovascular interventions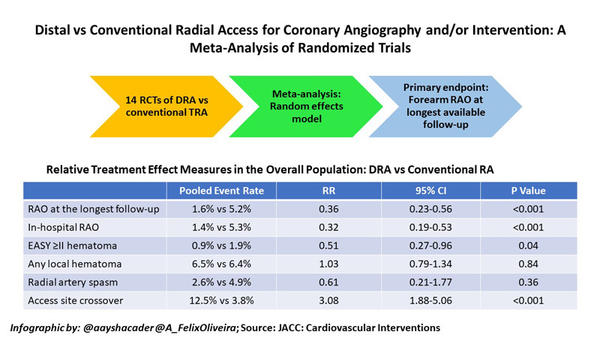
Critical reading and the relevance for clinical practice
This updated meta-analysis provides a comprehensive synopsis of currently available RCT data of DRA vs TRA on predominantly access-related endpoints, showing reduced risk of RAO and haematomas with DRA, albeit with increased time and attempts to secure access.
These results need to be interpreted in view of the fact that the study populations across many of the RCTs included mainly consisted of patients undergoing chronic coronary syndromes, with the majority excluding ACS (only 5 studies with ACS patient data available), and only a limited number enrolling patients undergoing PCI (8 studies with data available). Thus, the applicability of these results in these important subsets of patients remains a question.
The pooled RAO rates of conventional TRA still remain slightly higher than the threshold of < 5 % recommended as a quality metric by international consensus2. This is likely due to the lack of systematic implementation of best practices for RAO prevention, especially patent haemostasis, even across many RCTs, which may have magnified the beneficial effect of DRA. There was also variation across trials in factors known to influence the risk of RAO such as sheath size, anticoagulation, and spasmolytic used, as well as compression methods, with many studies reporting manual compression.
Nevertheless, theoretically, the anatomical location of DRA by virtue of being distal to the superficial palmar arch has the advantage of avoiding flow interruption in the forearm radial artery and reducing RAO3. The distal radial artery’s smaller diameter, distal location, angulation, and tortuosity predispose it to higher failure rates and access site crossover, which is technically more challenging and has a steeper learning curve8. Indeed, operators enrolling patients in these RCTs might naturally be more adept at conventional TRA access than DRA, which might have influenced results in favour of conventional TRA with respect to rapidly securing access and sheath insertion.
Considering all these aspects, while there does not appear to be sufficient data to recommend DRA as routine practice in all patients, it should be considered to reduce the risk of RAO in the subset of patients requiring preservation of radial artery patency such as those requiring AVF, CABG with radial arterial grafts and downstream repeat catheterisation.
References
- Rashid M, Kwok CS, Pancholy S, Chugh S, Kedev SA, Bernat I, et al. Radial Artery Occlusion After Transradial Interventions: A Systematic Review and Meta-Analysis. J Am Heart Assoc [Internet]. 2016 Jan 1 [cited 2022 Mar 11];5(1). Available from: https://pubmed.ncbi.nlm.nih.gov/26811162/
- Bernat I, Aminian A, Pancholy S, Mamas M, Gaudino M, Nolan J, et al. Best Practices for the Prevention of Radial Artery Occlusion After Transradial Diagnostic Angiography and Intervention: An International Consensus Paper. Cardiovasc Interv [Internet]. 2019 Nov 25 [cited 2022 Jul 19];12(22):2235–46. Available from: https://www.jacc.org/doi/10.1016/j.jcin.2019.07.043
- Sgueglia GA, Di Giorgio A, Gaspardone A, Babunashvili A. Anatomic Basis and Physiological Rationale of Distal Radial Artery Access for Percutaneous Coronary and Endovascular Procedures. JACC Cardiovasc Interv. 2018 Oct 22;11(20):2113–9.
- Kiemeneij F. Left distal transradial access in the anatomical snuffbox for coronary angiography (ldTRA) and interventions (ldTRI). EuroIntervention. 2017 Sep 1;13(7):851–7.
- Ferrante G, Condello F, Rao S V., Maurina M, Jolly S, Stefanini GG, et al. Distal vs Conventional Radial Access for Coronary Angiography and/or Intervention: A Meta-Analysis of Randomized Trials. Cardiovasc Interv [Internet]. 2022 Nov 28 [cited 2022 Nov 29];15(22):2297–311. Available from: https://www.jacc.org/doi/10.1016/j.jcin.2022.09.006
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ [Internet]. 2003 Sep 4 [cited 2022 Aug 30];327(7414):557–60. Available from: https://www.bmj.com/content/327/7414/557
- Lucreziotti S, Persampieri S, Gentile D, Barbieri L, Salerno-Uriarte D, Valli F, et al. Access-site hematoma in distal and conventional transradial access: a randomized trial. Minerva Cardiol Angiol [Internet]. 2022;70(2):129‐137. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02391181/full
- Roh JW, Kim Y, Lee OH, Im E, Cho DK, Choi D, et al. The learning curve of the distal radial access for coronary intervention. Sci Reports 2021 111 [Internet]. 2021 Jun 24 [cited 2022 Nov 29];11(1):1–7. Available from: https://www.nature.com/articles/s41598-021-92742-7