Edoxaban for thromboembolism prevention in pediatric patients with cardiac disease
Selected in JACC by L. Biasco
Pediatric patients requiring anticoagulation are a rare but extremely complex subgroup, with congenital (univentricular circulation, Fontan circulation, venous-to-systemic shunts) or acquired diseases (Kawasaki disease), which represents a therapeutic and logistic challenge.
References
Authors
Michael A. Portman, Jeffrey P. Jacobs, Jane W. Newburger, Felix Berger, Michael A. Grosso, Anil Duggal, Ben Tao, Neil A. Goldenberg, and on behalf of the ENNOBLE-ATE Trial Investigators
Reference
J Am Coll Cardiol. Oct 31, 2022. Epublished DOI: 10.1016/j.jacc.2022.09.031
Published
31 October 2022
Link
Read the abstractReviewer
Latest contributions
TAVI complications - Part 5 Unusual structural interventions: infective endocarditis, ventricular septal rupture & hypertrophic obstructive cardiomyopathy Tendyne transcatheter mitral valve system in patients with severe mitral annular calcification: one-year outcomes from the SUMMIT Severe MAC cohortMy Comment

PICOT Scheme - Courtesy of Luigi Biasco @BiascoDr, Source: PCRonline.com
Why this study – the rationale/objective?
Currently, subcutaneous weight-adjusted low molecular weight heparin (LMWH) with TID administration and vitamin K antagonists (AVK) have been used as a standard of care. Clearly, this approach adds complexity to complexity requiring repeated subcutaneous injections with LMWH and blood sampling in AVK-treated children to deliver therapy and monitor the efficacy of a treatment with a narrow therapeutic window.
Clearly, direct oral anticoagulant (DOAC) with single daily administration represents a potential alternative to current practice, nonetheless, no evidence supporting safety and efficacy of DOAC treatment in pediatric patients with predisposing cardiac conditions requiring primary or secondary prophylaxis of thromboembolic events is currently available.
How was it executed – the methodology?
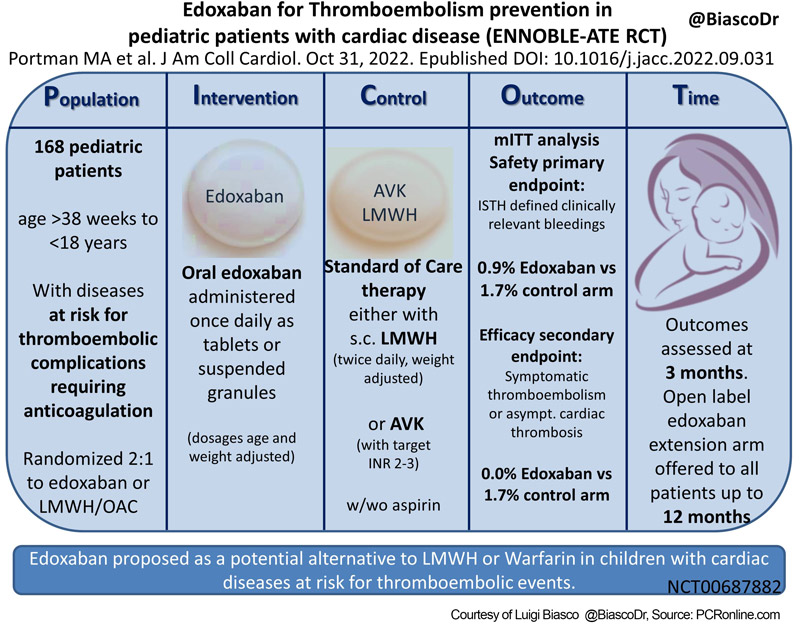
- International, prospective, open-label, randomized clinical trial.
- 2:1 randomization
- Treatment: Edoxaban administered once daily as tablets or suspended granules (dosages age and weight adjusted; e.g.: patients > 12 and < 18 years: 60 mg/OD for weight > 60 kg, 45 mg/OD for 30-60 kg, 30 mg/die if body weight below the 5th percentile for age; oral suspension 1,2-1,5 mg/kg for a maximum of 45 mg for patients with age < 6 years)
- Control: standard of care with either LMWH (twice daily, weight-adjusted doses), or AVK (with target INR 2-3), with or without concomitant treatment with aspirin.
- Primary safety endpoint: ISTH defined clinically relevant bleedings (major + clinically relevant non-major bleedings).
- Secondary efficacy endpoint: composite of symptomatic thromboembolism or asymptomatic cardiac thrombosis.
- Primary and secondary endpoints assessed at 3 months according to a modified Intention to Treat analysis (mITT). Following the initial study period, an open-label Edoxaban extension arm was offered to all patients.
- Outcomes assessed by an independent Data and Safety monitoring board.
- Due to the low sample size and rarity of events observed, only descriptive analyses are provided.
- Trial funded by Daiichi Sankyo, Inc.
What is the main result?
- 168 patients randomized across 48 centers (US, Canada, EU, Middle East) in 40 months.
- Treatment group: 110 pts, 109 effectively treated.
- Control group: 58 pts (55 AVK; 3 LMWH).
- 147 continued in the open-label Edoxaban extension arm.
- Prevalent predisposing cardiac conditions: Fontan circulation 44 %, Kawasaki disease 22 %.
- Suboptimal mean time in therapeutic range in the control arm: AVK 45 %; LWMH 79 %.
- Primary endpoint: 1 event (epistaxis) in the Edoxaban (0.9 %, annualized rate 0.037 events/100 patients-year) and 1 (hematochezia) in the control arm (1.7 %, annualized rate 0.069 events/100 patients-year). No major bleedings observed.
- Secondary endpoint: 0 event in the Edoxaban and 1 patient with events (Deep vein thrombosis and pulmonary embolism) in the control arm (1.7 %, annualized rate 0.07 events/100 patients-year).
- Open-label Edoxaban extension period: 2 bleedings (1 traumatic liver laceration and 1 hemorrhagic stroke) and 4 thromboembolic events (2 strokes and 2 coronary thromboses).
Critical reading and relevance for clinical practice
While faultless from a methodological perspective with well-defined inclusion criteria and pre-specified adjudicated endpoints, this study has to be analyzed and interpreted in light of the rarity of the condition and complexity of the setting that characterizes pediatric cardiology.
Clearly, the low sample size, the short follow-up time, and the low event rate observed in the study period preclude any evaluation of non-inferiority or superiority of treatment. Probably, we should refrain from the desire to use assessment criteria that apply to studies focusing on adult populations.
The high numbers of thromboembolic and hemorrhagic events observed within a short follow-up and in a relatively small cohort of patients (with the obvious clinical consequences and impact on quality of life of children and families) justify any effort to search for adequate therapeutic alternatives.
The lack of apparent harm associated with Edoxaban treatment as compared to current standard of care, as well as its apparent efficacy in preventing embolic events, are hypothesis-generating, and open the possibility for a newer, easy-to-administer, therapeutic approach that tries to simplify the complexity associated with pediatric cardiac conditions predisposing to thromboembolism.
The relevance of the hypotheses generated by this study is exemplified by the role of DOAC beyond TE/DVT and AF. This potential research opportunity is supported by the large numbers of patients with Kawasaki disease enrolled in the trial with giant coronary aneurysm requiring thromboembolic prophylaxis to prevent coronary thrombosis. Indeed, according to current practice, even adult patients presenting with giant coronary aneurysm are usually treated with AVK according to pieces of evidence mainly derived from retrospective multicenter registries, while no RCT-derived data are currently available.
Thus, even for this reason, we need to thank the authors of the ENNOBLE-ATE RCT for their efforts in collecting those rare patients among numerous international centers over more than 3 years and for the courage shown in evaluating this potential therapeutic alternative with falls out far beyond pediatric cardiology.




No comments yet!