Haemodynamic performance and clinical outcomes of transcatheter aortic valve replacement with the self-expanding ACURATE Neo2
Selected in EuroIntervention by B. Hennessey , N. Ryan
The ACURATE neo2 valve was developed to reduce the risk of paravalvular AR with an increased sealing skirt. This retrospective study aimed to compare TAVI with the ACURATE neo and neo2 devices.
References
Authors
Andrea Scotti, Pagnesi, Won-Keun Kim, Ulrich Schäfer, Marco Barbanti, Giuliano Costa, Sara Baggio, Matteo Casenghi, Federico De Marco, Maarten Vanhaverbeke, Lars Søndergaard, Alexander Wolf, Joachim Schofer, Marco Bruno Ancona, Matteo Montorfano, Ran Kornowski, Hana Vaknin Assa, Stefan Toggweiler, Alfonso Ielasi, David Hildick-Smith, Stephan Windecker, Albrecht Schmidt, Andrea Buono, Diego Maffeo, Dimytri Siqueira, Francesco Giannini, Marianna Adamo, Mauro Massussi, David A. Wood, Jan-Malte Sinning, Jan Van der Heyden, Dirk-Jan van Ginkel, Nicholas Van Mieghem, Verena Veulemans, Darren Mylotte, Vasileios Tzalamouras, Maurizio Taramasso, Rodrigo Estévez-Loureiro, Antonio Colombo, Antonio Mangieri, Azeem Latib
Reference
June 8, 2022 | 10.4244/EIJ-D-22-00289
Published
8 June 2022
Link
Read the abstract
Reviewers
Our Comment
Why this study - the rationale / objective:
Moderate to severe paravalvular aortic regurgitation post-TAVI is known to be associated with poorer outcomes in the medium and long term.
Given the expanding indications for TAVI, minimising complications and improving long-term clinical outcomes is of utmost importance.
Previous trials have shown increased paravalvular AR with the ACURATE neo valve1,2, particularly in patients with increased device landing zone calcification.
The ACURATE neo2 valve was developed to reduce the risk of paravalvular AR with an increased sealing skirt.
This retrospective study aimed to compare TAVI with the ACURATE neo and neo2 devices.
How was it executed? - the methodology:
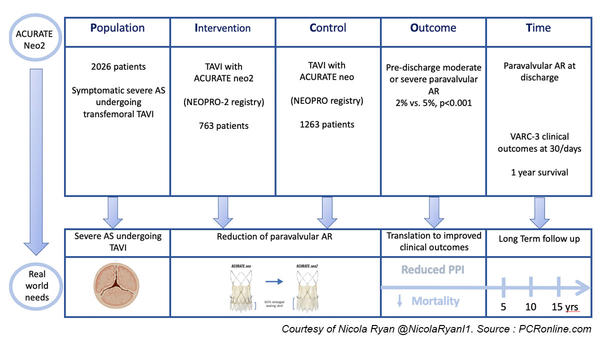
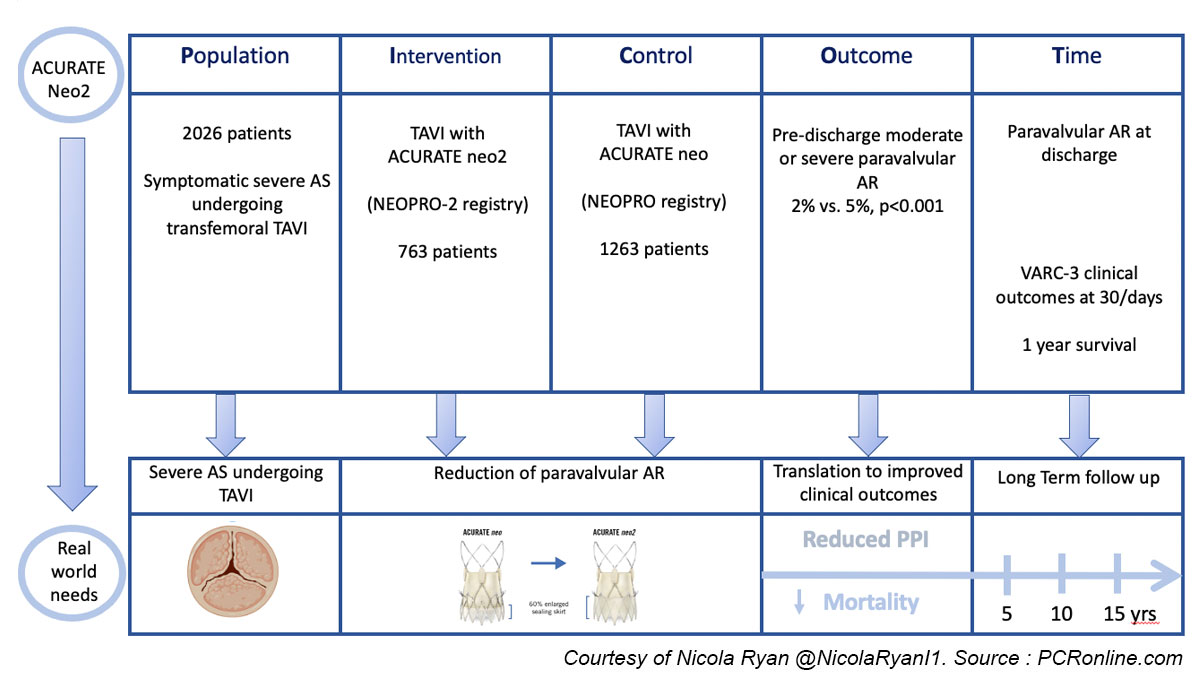
The NEOPRO registry included consecutive patients undergoing clinically indicated transfemoral TAVI with the ACURATE neo valve in 24 centres, from Jan 2012-March 2018.
Subsequently, the NEOPRO-2 registry included patients in whom an ACURATE neo2 valve was implanted in 20 centres.
This study analysed data from 29 centres implanting the ACURATE neo (18) and neo2 (16) devices between Jan 2012-March 2018 and Sept 2020-Dec 2021 respectively.
- The primary endpoint was moderate or severe paravalvular AR at predischarge transthoracic echocardiography
- Secondary endpoints were VARC-3 defined clinical outcomes at 30 days which include technical and procedural success, pacemaker implantation bleeding, and vascular complications
- 1-year survival was a secondary endpoint
What is the main result?
Overall, 2,026 patients, 1,263 undergoing TAVI with the ACURATE neo and 763 with the ACURATE neo2 were included in this study.
The majority of patients were women with a mean age of 82 years, in keeping with the expanding indications for TAVI, the EuroSCORE II and STS score were higher in the ACURATE neo group, and more patients presented in NYHA III/IV in the ACURATE neo group.
The ACURATE neo2 group, more commonly, had mild and moderate LVOT calcification with more severe AV calcification in the ACURATE neo group.
There were no significant differences in the valve sizes between the groups.
- There was significantly less pre-discharge moderate-severe paravalvular AR in the neo2 group (2 % vs 5 %, p < 0.001).
- When analysed by aortic valve calcification, patients with heavy AV calcification had a significant reduction in moderate or severe paravalvular AR with the ACURATE neo2 compared to the ACURATE neo (2 % vs. 9 %, p = 0.018).
- At 30 days, there were no significant differences in pacemaker implantation rates between groups [9 % (neo) vs. 8 % (neo), p = 0.46].
- Mean aortic gradients were higher in the neo2 group (8.9 ± 4.1 vs 8 ± 3.3, p < 0.001) though there were no significant differences between AVA or indexed AVA.
- Both bleeding (p = 0.02) and vascular (p < 0.001) complications were lower in the neo2 group.
- There was no significant difference in all-cause mortality at one-year between groups [90 % (neo2) 95 %CI 83-98 vs. 87 % (neo) 95 %CI 84-90, p = 0.14].
Critical reading and the relevance for clinical practice:
The results of this study show that, using real-world registry data, there were significantly less moderate to severe paravalvular AR in patients treated with the ACURATE neo2 valve compared to the ACURATE neo valve.
This reduction in moderate to severe paravalvular AR was particularly demonstrated in patients with severe native valve calcification.
Importantly, as the indications for TAVI expand, both vascular and bleeding complications were lower with the ACURATE neo2 valve. This is likely multifactorial, with increased operator experience with both valve implantation and management of access sites, as well as evolution of the introducer likely contributing.
It must be borne in mind that there is a number of limitations to this study, including the registry-based nature of the study, as well as the lack of core lab adjudication.
Furthermore, valves were implanted over an almost ten-year period across the two registries.
The evidence from previous RCT’s had raised questions with regard to the role of the ACURATE neo valve, particularly in patients with heavily calcified native valve.
Whilst this data is non-randomised and did not show a significant difference in 1-year survival, the evolution of the ACURATE neo2 valve appears to have addressed the issue of moderate to severe paravalvular leak in the short term.
Whether this reduction in paravalvular AR translates to improved longer-term clinical outcomes is yet to be determined.
The results of the ongoing RCT and registry investigating the ACURATE neo2 will provide contemporaneous evidence with regard to the performance of the valve.
References
- Tamburino C, Bleiziffer S, Thiele H, Scholtz S, Hildick-Smith D, Cunnington M, et al. Comparison of Self-Expanding Bioprostheses for Transcatheter Aortic Valve Replacement in Patients With Symptomatic Severe Aortic Stenosis. Circulation. 2020 Dec 22;142(25):2431–42.
- Lanz J, Kim WK, Walther T, Burgdorf C, Möllmann H, Linke A, et al. Safety and efficacy of a self-expanding versus a balloon-expandable bioprosthesis for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: a randomised non-inferiority trial - The Lancet - Published: September 27, 2019 DOI:https://doi.org/10.1016/S0140-6736(19)32220-2







No comments yet!