Prevention of radial artery occlusion of 3 hemostatic methods in transradial intervention for coronary angiography
Selected in JACC: Cardiovascular Interventions by A. Cader
The main objective of the PROTHECT (Prevention of Radial artery Occlusion of Three HEmostatiC methods in Transradial intervention for coronary angiography) trial was to compare the efficacy of 3 nonocclusive haemostatic methods in preventing early RAO2.
References
Authors
Guering Eid-Lidt, Jesús Reyes-Carrera, Julio I. Farjat-Pasos, Arnoldo Loaisiga Saenz, Carlos Aguila Bravo, Sara Nieto Rangel, Daniel Zazueta Salido, Norman Said Vega Servin, Maria Elena Soto-López, and Jorge Gaspar
Reference
J Am Coll Cardiol Intv. 2022 May, 15 (10) 1022–1029
Published
15 May 2022
Link
Read the abstract
Reviewer
Latest contributions
Highlights of science and education in cardiovascular interventional medicine in 2025 OCT insights of thrombus extraction by stent-retriever thrombectomy (SRT) and manual aspiration (MA): Insights from RETRIEVE AMI trial Randomised comparison of intravascular lithotripsy vs. cutting balloon treatment in calcified coronary artery disease – The Short-CUT trialMy Comment
Why this study – the rationale/objective?
Radial artery occlusion (RAO) is the most frequent complication of the transradial approach1. Haemostasis methods, arterial compression time, and level of compression are important non-pharmacological factors that modify incidence of RAO.
The main objective of the PROTHECT (Prevention of Radial artery Occlusion of Three HEmostatiC methods in Transradial intervention for coronary angiography) trial was to compare the efficacy of 3 nonocclusive haemostatic methods in preventing early RAO2.
- Standard patent haemostasis (PH): via pneumatic TR band
- Patent haemostasis with ulnar compression or the ulnar artery transient compression facilitating radial artery patent haemostasis (ULTRA) method (UM)
- Facilitated haemostasis (FH): with a stat seal haemostatic device (HD) and TR band placed over it
All these three methods have been proven by randomised controlled trials to reduce RAO (3-5), but not been previously compared against each other for RAO.
How was it executed - the methodology
PICOT criteria:
Population: Inclusion criteria: Consenting patients ≥ 18 years old in whom successful radial access was obtained for either a coronary diagnostic or therapeutic procedure were included.
Exclusion criteria:
• Recent transradial procedure in the same radial artery (<1 month)
• Failed transradial access
• Crossover of the initial access
• Patients in cardiogenic shock
Interventions & comparators:
Three non-occlusive haemostasis methods mentioned above were compared, randomised 1:1:1 by block randomisation.
Study procedures:
Routine conventional transradial access was achieved by the Seldinger technique.
A cocktail of 5,000 IU of unfractionated heparin (UFH), 2.5 mg verapamil, and 200 mg nitroglycerin was administered through the sheath. Additional UFH was given for PCI at a dose of 100U/kg.
At completion of procedure, haemostasis in each arm was achieved as per protocol.
Endpoints:
The primary endpoint was RAO at 24 hours, for which the study was powered.
The secondary endpoints were vascular complications and haemorrhagic complications such as major bleeding, minor bleeding, and hematomas according to the EASY (Early discharge After transradial tenting of coronary arteries) classification.
Follow-up and RAO:
Radial artery patency was evaluated at 24 hours after sheath removal with oximetry plethysmography.
All RAO detected by plethysmography were corroborated by Doppler ultrasound, and repeated at 30 days if positive for RAO by ultrasound at 24 hours.
Although not mentioned in the main paper, the Clinicaltrials.gov entry indicates blinding at the outcomes-assessor level, with the investigator evaluating the primary endpoint being blinded to the haemostatic technique the patient received.
What is the main result?
A total of 1,470 patients were randomised. A single patient in the PH group was lost to follow-up, so 1,469 patients were analysed: 491 patients in group 1 with PH, 490 patients in group 2 with the UM, and 488 patients in group 3 with facilitated haemostasis with an HD.
Demographic variables were comparable across the 3 groups. The mean age was 60.3 ± 11.1 years. Just under three-quarters of the population were male. The right radial artery was the most frequent access site (93.1 %).
Overall, successful radial haemostasis was achieved in 94.4 % of the procedures. Diagnostic studies versus interventional procedures were 47.4 % and 52.6 %, respectively, which were comparable across the three groups.
85 % of the entire study group received UFH doses between 5,000 and 10,000. The average haemostasis time was 132.9 ± 55.6 minutes overall.
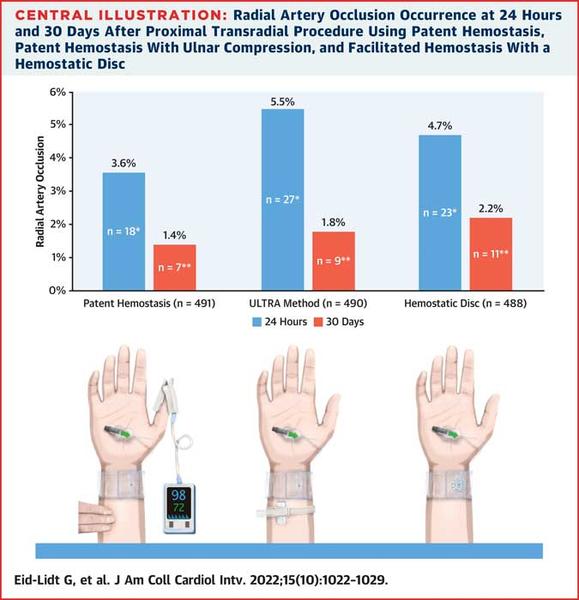
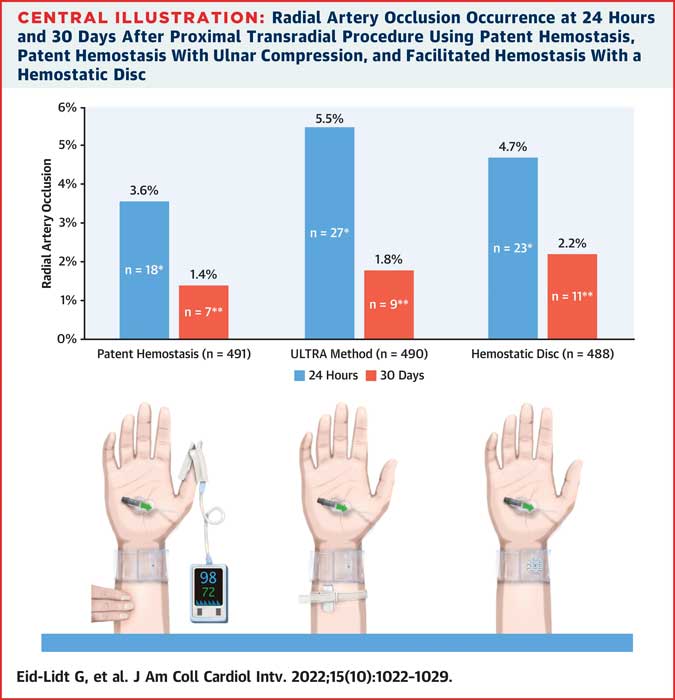
Radial Artery Occlusion rates at 24 hours and 30 days across the non-occlusive haemostasis arms in the PROTHECT trial
Source = JACC: Cardiovascular Interventions
Primary endpoint:
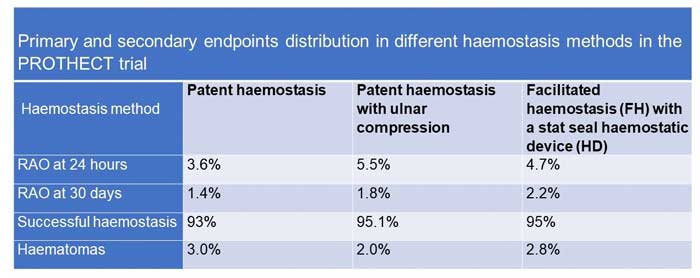
Overall, RAO at 24 hours was 4.6 %. There were no significant differences in 24-hour RAO between the 3 haemostasis groups: 3.6 % in the PH group, 5.5 % in the UM group, and 4.7 % in the HD group (P = 0.387).
Overall, RAO at 30 days was 1.8 %, also with no significant differences between groups: 1.4 % in the PH group, 1.8 % in the UM group, and 2.2 % in the HD group (P = 0.185).
Secondary endpoints:
Vascular complications and bleeding occurred at 2.9 %. Overall, bleeding occurred in 2.79 %, with no significant differences across the groups (p = 0.55). Haematomas occurred in 2.6 % overall, which did not change even at UFH doses > 10,000 U (2.8 %, p = 0.55).
The independent predictors of RAO prevention at 24 hours were the use of hydrophilic coating introducers (OR: 0.46; 9 5% CI: 0.27-0.76; P = 0.003) and interventional procedures (OR: 0.47; 95% CI: 0.27-0.81; P = 0.007), possibly owing to extra doses of heparin.
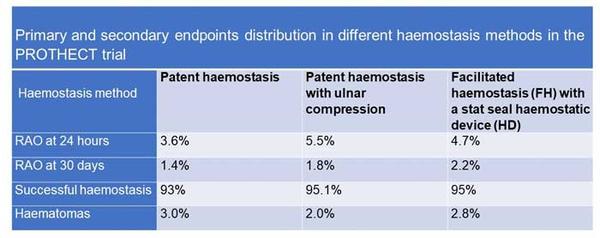
Primary and secondary endpoints distribution across the non-occlusive haemostasis arms in the PROTHECT trial
Source = JACC: Cardiovascular Interventions
Critical reading and the relevance for clinical practice
This trial found no differences in three methods of haemostasis in preventing RAO at 24 hours and 30 days. All methods were non-occlusive haemostasis.
Contemporary best practices have resulted in significant reduction of RAO rates1. These include non-occlusive haemostasis methods as in this trial, but also optimizing anticoagulation and reducing vascular damage (i.e. with the use of hydrophilic sheaths, used in ~ 80 % in this trial).
The non-occlusive methods in this trial have all been proven by randomised controlled trials to be superior to conventional haemostasis. Specific comparisons of RAO rates in each arm of the PROTHECT trial with the most important trial for each method are made below:
Comparison of each arm with prior RCTs:
Patent haemostasis: Comparable RAO rates were seen between the patent haemostasis arm of this PROTHECT trial and the landmark PROPHET I trial (3.6 % vs 5.4 % at 24 hours, 1.4 % vs 1.8 % at 30 days, for PROTHECT vs PROPHET I respectively)3. However, unlike the present study, PROPHET I only included diagnostic angiographies, and not PCI3.
Patent haemostasis with ulnar compression: RAO rates in this arm were higher than those reported in the PROPHET II trial, which compared patent haemostasis with ipsilateral ulnar artery compression (24-hour RAO 5.5% vs 1.0% and 30-day RAO 1.8 % vs 0.9 % for PROTHECT vs PROPHET II respectively)4. This has been attributed to the greater expertise in centres that recruited I PROPHET II.
Facilitated haemostasis (FH) with a stat seal haemostatic device (HD): Higher RAO rates were observed in PROTHECT than in the STAT trial5, wherein a potassium ferrate haemostatic patch (PFHP) was used for a rapid deflation protocol (4.7 % versus 2.0 % for PROTHECT vs STAT respectively). This can be explained by the prolonged haemostasis time in PROTHECT which was almost triple than observed in STAT. In contrast, for the same reasons of prolonged haemostasis, haematomas were much fewer in PROTHECT HD arm than in STAT (2 % vs 17.2 %).
In terms of limitations, duplex ultrasound for RAO was only performed in those who demonstrated RAO by plethysmography, potentially missing some occlusions. A 50 % reduction of RAO in the experimental groups seems like an over-optimistic effect size, which was used for sample size calculation. Furthermore, the fact that this was a single centre trial somewhat limits its generalisability and external validity.
Despite the evidence, we know little of real-world global trends in best practice of non-occlusive haemostasis methods.
What is your current practice?
References
- Bernat I., Aminian A., Pancholy S., et al. "Best practices for the prevention of radial artery occlusion after transradial diagnostic angiography and intervention. An international consensus paper". J Am Coll Cardiol Intv 2019;12:2235-2246.
- Eid-Lidt G, Reyes-Carrera J, Farjat-Pasos JI, et al. Prevention of Radial Artery Occlusion of 3 Hemostatic Methods in Transradial Intervention for Coronary Angiography. JACC Cardiovasc Interv. 2022 May 23;15(10):1022-1029. doi: 10.1016/j.jcin.2022.03.011. PMID: 35589232.
- Pancholy S., Coppola J., Patel T., Roke-Thomas M. "Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET Study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization". Catheter Cardiovasc Interv 2008;72:335-340.
- Pancholy S., Bernat I., Bertrand O., Patel T. "Prevention of radial artery occlusion after transradial catheterization. The PROPHET II randomized trial". J Am Coll Cardiol Intv 2016;9:1992-1999.
- Seto A., Rollefson W., Patel M., et al. "Radial haemostasis is facilitated with a potassium ferrate haemostatic patch: the Statseal with TR Band assessment trial (STAT)". EuroIntervention 2018;14:e1236-e1242.




No comments yet!