Short-term postoperative use of Rivaroxaban to prevent radial artery occlusion after transradial coronary procedure: the RESTORE randomized trial
Selected in Circulation: Cardiovascular Interventions by A. Cader , S. R. Khan
The RESTORE trial aimed to evaluate the clinical value of short-term postoperative anticoagulation with Rivaroxaban 10 mg daily for 7 days as compared with placebo, in reducing RAO at 24 hours and 30 days.
References
Authors
Dongjie Liang, Qingcheng Lin, Qianli Zhu, Xiaodong Zhou, Ying Fang, Liangguo Wang, Guangze Xiang, Kenneth I. Zheng, Weijian Huang, and Peiren Shan
Reference
Circulation: Cardiovascular Interventions. 2022;0:CIRCINTERVENTIONS.121.011555
Published
23 March 2022
Link
Read the abstract
Reviewers
Our Comment
Why this study – the rationale/objective?
Radial Artery Occlusion (RAO) remains the Achilles’ Heel of transradial intervention. Thrombus formation is the principal mechanism of RAO after a transradial coronary procedure. There is randomised evidence that adequate procedural anticoagulation is crucial for RAO prevention1,2.
However, the effect of postprocedural anticoagulation in reducing RAO following transradial approach (TRA) has not been investigated. The RESTORE trial aimed to evaluate the clinical value of short-term postoperative anticoagulation with Rivaroxaban 10 mg daily for 7 days as compared with placebo, in reducing RAO at 24 hours and 30 days3.
How was it executed? - the methodology
The RESTORE trial was a randomized, parallel-arm, placebo-controlled, single-centre clinical trial performed at the First Affiliated Hospital of Wenzhou Medical University, China.
Population - Inclusion criteria:
Patients scheduled for clinically indicated diagnostic or interventional coronary procedures via TRA were enrolled. Of note, pre-procedural patency of radial artery was confirmed by duplex ultrasound (DUS). Only 6 French sheaths were used.
Exclusion criteria:
- oral anticoagulation as current therapy for other indications;
- high bleeding risk according to CRUSADE score;
- hemodynamic instability;
- failed of radial artery puncture;
- and contraindication to the use of Rivaroxaban.
TRA procedure:
Access was achieved via 6 French standard sheaths only. All patients were given 200 µg intraarterial nitroglycerin. 2,500 IU of unfractionated heparin was given for diagnostic angiographies. Up to 100 IU/ kg of UFH was given for ad hoc intravascular ultrasounds or PCI, to maintain activated clotting time of ~250 seconds.
A haemostatic compression device was applied at the completion of transradial procedure, however NO patent haemostasis protocol was followed. All patients were pre-treated with dual antiplatelet therapy (DAPT).
Intervention & Comparator:
Patients were then randomised 1:1 to receive either placebo (control arm) or Rivaroxaban 10 mg once daily for 7 days (intervention arm) by simple randomisation. The choice of Rivaroxaban dose (10 mg daily) was selected based on its proved efficacy and tolerability for the prevention of venous thromboembolism.
Endpoints:
The primary endpoint was early RAO (at 24 hours): this was evaluated by duplex Doppler Ultrasound (DUS) within 24 hours of removal of compression device by operators who were blinded to the randomisation allocation.
The secondary endpoint was late RAO, assessed at 30 days by DUS.
A safety endpoint of bleeding was defined as per Bleeding Academic Research Consortium (BARC).
Statistical analysis:
The study was powered at 80 % with a 2-sided alpha of 0.05 to detect a reduction of RAO incidence with an estimated incidence rate of 10 % in the control arm and 3 % in the intervention arm. The calculated sample size was 382.
What is the main result?
382 patients underwent randomisation, 191 in each arm. The mean age was ~ 64 years; 63.6 % were men. Almost half of the procedures included ad hoc IVUS or PCI, requiring more anticoagulation.
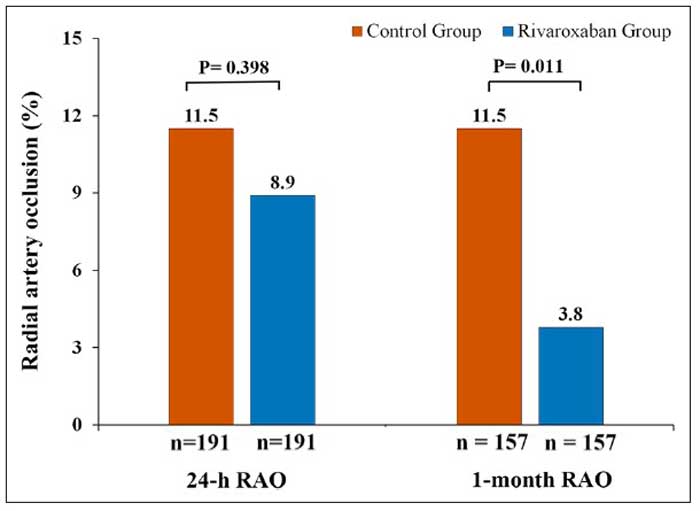
The primary endpoint, RAO at 24-hours occurred in 8.9 % in the Rivaroxaban arm versus 11.5 % in the placebo arm (odds ratio, 0.75 [95 % CI, 0.39–1.46]; P = 0.399).
Although no differences between the two arms were noted for early RAO, RAO at 1 month (N = 157 in each group) was significantly lower in the Rivaroxaban arm compared with the control arm (3.8 % vs 11.5 %; P = 0.011). All RAO were asymptomatic. The recanalization rate of the radial artery at 30 days was significantly higher in the Rivaroxaban arm (69.2 %), as compared with 30.0 % in patients of the control arm (P = 0.027).
There were no differences between the groups in terms of the safety endpoint: (BARC bleeding type 1: 2.6 % vs 4.7 %; P = 0.276 or BARC type 2 bleeding 0.5 % vs 2.1 %; P = 0.372, for placebo versus rivaroxaban arms respectively). No differences were seen in EASY type 1 forearm hematomas either (2.1 % and 1.0 % in control vs Rivaroxaban arms respectively; p = 0.685). No other access site complications were observed.
Rivaroxaban was an independent negative predictor of 1-month RAO at 30 days on multivariate logistic regression analysis (OR, 0.22 [95 % CI, 0.08 – 0.65]; p = 0.006), and remained so when repeated with multiple imputation for missing data.
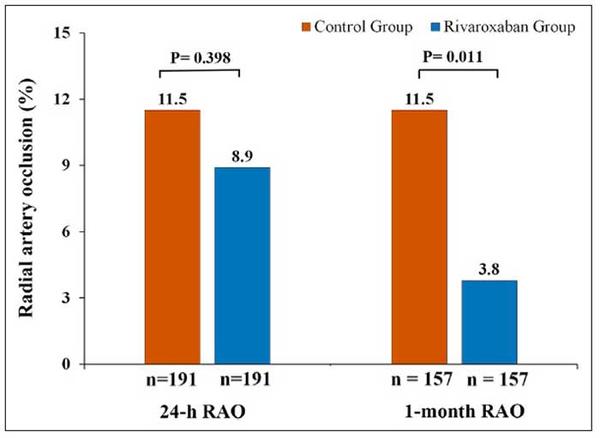
Radial artery occlusion (RAO) at 24-hours and 1-month post-transradial access procedure
Source = Circulation: Cardiovascular Interventions
Critical reading and the relevance for clinical practice?
The RESTORE trial showed that short-term postoperative anticoagulation with Rivaroxaban did not reduce the rate of 24-hour RAO but improved 1-month RAO, possibly owing to higher recanalization of the radial artery4.
Importantly, this was not accompanied by excess bleeding. Of note, however, is that a number of randomised trials of RAO, including a 2016 meta-analysis, have also reported a reduced incidence of RAO at 30 days, without additional intervention5,6.
In the SPIRIT OF ARTEMIS randomised trial, a peri-procedural higher heparin dose (100 IU/kg) was shown to significantly reduce the rate of early RAO (median of 2 days) compared with standard dose (50 IU/kg) (3.0 % vs 8.1 %; OR: 0.35; 95 % CI: 0.22 to 0.55; p < 0.001), without compromising safety. This trial excluded PCI patients, and included either 5- or 6-F coronary angiography1.
The findings of RESTORE trial suggest that 10 mg Rivaroxaban once daily postprocedural may not be sufficient to prevent RAO at 24 hours, prompting the question of perhaps the need to test this effect with a higher dose. However, this dose was sufficient to reduce 1-month RAO, and may potentially be associated with higher recanalization. Having said that, this trial was not designed to investigate the treatment effect of rivaroxaban for early RAO, although the use of low molecular weight heparin for symptomatic RAO has been reported as a treatment option6.
Another important fact is the relatively higher rates of RAO in this study, in both arms, especially in comparison to contemporary trials. This could be partially explained by the non-application of patent haemostasis, exclusive use of the larger 6 French sheath, and varied duration for postprocedural radial compression, with relatively longer the haemostatic time possibly due to delayed sealing of puncture site caused by DAPT. All participants were pre-loaded with DAPT, a pre-requisite that is not easily explained, especially given that half of them were also randomised to anticoagulation.
Although the trial is single-centre, which limits its generalizability, the RAO incidence is a reflection of current practice in many busy centres, where often patent haemostasis and ipsilateral ulnar compression are not practised routinely in radial haemostasis protocols. Indeed, the trial was also powered at an estimated incidence of 10 % RAO in the control group, and 3 % in the Rivaroxaban group, probably derived from the SPIRIT OF ARTEMIS data1.
The percentage of women enrolled in this trial was 36.4 %. While female sex has been shown to be a predictor of RAO7, pre-specified sub-group analyses of this trial found that Rivaroxaban was associated with a lower incidence of early RAO in female participants, as compared with placebo (OR, 0.28; 95 % CI, 0.08–0.93; p for interaction = 0.033), as well as those with diabetes (OR, 0.11; 95 % CI, 0.01–0.88; p for interaction = 0.011), another factor known to be at risk of RAO.
In this relatively small trial of 382 patients, RESTORE has opened a potential new dimension in RAO prevention with the introduction of post-procedural Rivaroxaban. Larger trials investigating different doses of Rivaroxaban are warranted.
In this respect, the currently recruiting Phase 3 trial CAPITAL-RAPTOR (Rivaroxaban Post-Transradial Access for the Prevention of Radial Artery Occlusion, NCT03630055), which randomises 1,800 patients to Rivaroxaban 15 mg and placebo, may have some answers.
References:
- Hahalis GN, Leopoulou M, Tsigkas G, , et al. Multicenter randomized evaluation of high versus standard heparin dose on incident radial arterial occlusion after transradial coronary angiography: the SPIRIT OF ARTEMIS Study. JACC Cardiovasc Interv. 2018;11:2241–2250
- Hahalis G, Aznaouridis K, Tsigkas G, , et al. Radial artery and ulnar artery occlusions following coronary procedures and the impact of anticoagulation: ARTEMIS (Radial and Ulnar ARTEry Occlusion Meta-AnalysIS) systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e005430.
- Bernat I, Aminian A, Pancholy S, et al; RAO International Group. Best Practices for the Prevention of Radial Artery Occlusion After Transradial Diagnostic Angiography and Intervention: An International Consensus Paper. JACC Cardiovasc Interv. 2019;12(22):2235-2246.
- Eid-Lidt G, Rivera Rodríguez A, Jimenez Castellanos J, et al. Distal radial artery approach to prevent radial artery occlusion trial. JACC Cardiovasc Interv. 2021;14:378–385.
- Rashid M, Kwok CS, Pancholy S, et al. Radial artery occlusion after transradial interventions: a systematic review and meta-analysis. J Am Heart Assoc. 2016;5:e002686. doi: 10.1161/JAHA.115.002686
- Zankl AR, Andrassy M, Volz C, et al. Radial artery thrombosis following transradial coronary angiography: incidence and rationale for treatment of symptomatic patients with low-molecular-weight heparins. Clin Res Cardiol. 2010;99:841–847.
- Bernat I, Aminian A, Pancholy S, et al; RAO International Group. Best Practices for the Prevention of Radial Artery Occlusion After Transradial Diagnostic Angiography and Intervention: An International Consensus Paper. JACC Cardiovasc Interv. 2019;12(22):2235-2246.





No comments yet!