Comparison of different percutaneous revascularisation timing strategies in patients undergoing transcatheter aortic valve implantation
Selected in EuroIntervention by A. Beneduce , O.A. Oliva
The aim of the REVASC-TAVI study was to compare different PCI timing strategies in patients with AS and significant CAD undergoing TAVI.
References
Authors
Tobias Rheude; Giuliano Costa; Flavio Luciano Ribichini; Thomas Pilgrim; Ignacio J. Amat Santos; Ole De Backer; Won‐Keun Kim; Henrique Barbosa Ribeiro; Francesco Saia; Matjaz Bunc; Didier Tchetche; Philippe Garot; Darren Mylotte; Francesco Burzotta; Yusuke Watanabe; Francesco Bedogni; Tullio Tesorio; Marco Tocci; Anna Franzone; Roberto Valvo; Mikko Savontaus; Hendrik Wienemann; Italo Porto; Caterina Gandolfo; Alessandro Iadanza; Alessandro S. Bortone; Markus Mach; Azeem Latib; Luigi Biasco; Maurizio Taramasso; Marco Zimarino; Daijiro Tomii; Philippe Nuyens; Lars Sondergaard; Sergio F. Camara; Tullio Palmerini; Mateusz Orzalkiewicz; Klemen Steblovnik; Bastien Degrelle; Alexandre Gautier; Paolo Alberto Del Sole; Andrea Mainardi; Michele Pighi; Mattia Lunardi; Hideyuki Kawashima; Enrico Criscione; Vincenzo Cesario; Fausto Biancari; Federico Zanin; Giovanni Esposito; Matti Adam; Eberhard Grube; Stephan Baldus; Vincenzo De Marzo; Elisa Piredda; Stefano Cannata; Fortunato Iacovelli; Martin Andreas; Valentina Frittitta; Elena Dipietro; Claudia Reddavid; Orazio Strazzieri; Silvia Motta; Domenico Angellotti; Carmelo Sgroi; Erion Xhepa; Faraj Kargoli; Corrado Tamburino; Michael Joner; Marco Barbanti
Reference
EuroIntervention 2023;19. DOI: 10.4244/EIJ-D-23-00186
Published
July 12, 2023
Link
Read the abstract
Reviewers
Our Comment
Why this study? – the rationale/objective
Significant coronary artery disease (CAD) is frequently observed in patients with severe aortic stenosis (AS) undergoing transcatheter aortic valve implantation (TAVI). Despite guidelines recommending percutaneous coronary intervention (PCI) of significant lesions in proximal segments in this population, the extent and the timing of revascularization are yet to be defined, as limited evidence is available1-3.
The aim of the study by Rheude et al. was to compare different PCI timing strategies in patients with AS and significant CAD undergoing TAVI4.
How was it executed? - the methodology
The REVASC-TAVI was an investigator-initiated, international, multicenter, retrospective registry including patients undergoing TAVI with concomitant stable CAD at pre-procedural work-up between January 2015 and September 2021 from 30 centers worldwide.
In this analysis of the study, patients undergoing revascularization were stratified according to PCI timing:
- PCI before TAVI, performed in a preceding elective procedure;
- PCI after TAVI, performed in a subsequent elective procedure;
- PCI concomitant with TAVI, performed in the same procedure.
Procedure timing and valve choice were at the operator’s discretion. International guidelines on myocardial revascularization were used to define significant CAD1.
The main endpoints were all-cause death and a composite of all-cause death, stroke, myocardial infarction or re-hospitalization for congestive heart failure at 2 years, as defined by Valve Academic Research Consortium (VARC)-2 criteria. To account for the non-randomized study design, outcomes were adjusted using the inverse probability treatment weighting (IPTW) method.
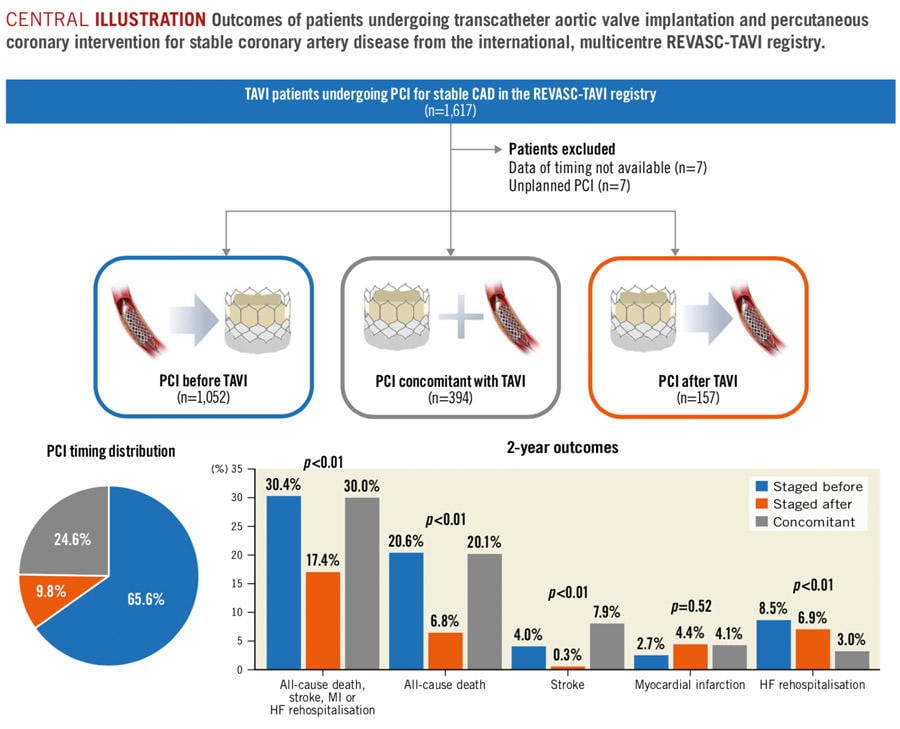
Central illustration: Outcomes of patients undergoing transcatheter aortic valve implantation and percutaneous coronary intervention for stable coronary artery disease from the international, multicentre REVASC-TAVI registry.
Source: EuroIntervention
What is the main result?
A total of 1,603 patients and 2,014 coronary lesions enrolled in the REVASC-TAVI registry were included in the study.
Baseline, CAD, PCI and TAVI characteristics
- The most adopted revascularization strategy was PCI before TAVI (65.6 % – median time frame 35 days), followed by PCI concomitant with TAVI (26.6 %), and PCI after TAVI (9.8 % – median time frame 40 days).
- After IPTW adjustment, baseline clinical and angiographic characteristics were balanced between the 3 groups, although the use of dual antiplatelet therapy (DAPT), dual antithrombotic therapy (DAT) or triple antithrombotic therapy (TAT) was significantly higher in patients undergoing PCI before TAVI, and patients undergoing PCI after TAVI presented with a significantly higher proportion of bifurcation lesions.
- Regarding PCI procedural characteristics, target vessel stenoses > 90 % and chronic total occlusions were more frequently treated before TAVI (p = 0.021), whereas left main or proximal left anterior descending artery lesions were less commonly treated after TAVI (p = 0.043 and p = 0.014 respectively). Radial approach was used only in 48.8 % of cases overall, most commonly for PCI before TAVI, while femoral access route was preferred for PCI concomitant with or after TAVI (p < 0.001). In patients receiving concomitant interventions, PCI was performed before valve implantation in 68.5 % of cases.
- Regarding TAVI procedural characteristics, valve type differed significantly among the 3 groups (p < 0.001), with balloon-expandable devices being preferred in patients undergoing PCI concomitant with or after TAVI. The need for post-dilatation was significantly higher in patients undergoing PCI before TAVI (p = 0.006). The contrast volume was significantly higher in case of PCI concomitant with TAVI (p < 0.001).
Clinical outcomes
- Patients undergoing PCI concomitant with TAVI experienced the highest rate of in-hospital mortality (p = 0.005), major vascular complications (p = 0.077), major bleeding (p = 0.025), and acute kidney injury (p = 0.011) as compared to those treated with PCI before or after TAVI.
- At 2 years, all-cause death was significantly lower in patients undergoing PCI after TAVI as compared with those receiving PCI before or concomitant with TAVI (6.8 % vs 20.1 % vs 20.6 %, p < 0.001). The composite endpoint was significantly lower in patients undergoing PCI after TAVI as compared with those receiving PCI before or concomitant with TAVI (17.4 % vs 30.4 % vs 30.0 %; p = 0.003). Results were confirmed at landmark analyses considering events from 0 to 30 days and from 31 to 720 days.
Critical reading and the relevance for clinical practice
Several considerations impact the decision on PCI timing in patients undergoing TAVI:
- clinical and anatomical relevance of CAD;
- concerns about the risk of bleeding, vascular access complications and contrast medium dose;
- concerns about hemodynamic or ischemic complications during valve implantation;
- concerns about the feasibility of coronary re-access after TAVI.
The present analysis of the REVASC-TAVI registry captures contemporary practice and clinical outcomes, providing important insights in this field.
Among possible revascularization strategies, PCI before TAVI or concomitant with TAVI resulted the most widely adopted ones (90.2 %). Nevertheless, although the 3 groups had similar CAD complexity and extension, a strategy of staged PCI after TAVI portended the best in-hospital and long-term clinical outcomes at 2 years follow-up, while PCI concomitant with TAVI seemed harmful despite potential logistic advantages.
Focusing on the pathophysiological mechanisms favoring staged PCI after TAVI, upfront correction of AS seems key, reducing left ventricular pressure overload, improving microvascular circulation and preventing transient hemodynamic compromise events that may cause renal or cerebral ischemia during PCI.
Interestingly, the observed effect of PCI timing on clinical outcomes could be also related to a complex interplay with its therapeutic implications (valve choice, vascular access route selection, contrast medium dose). The significantly higher proportion of balloon-expandable valves in the PCI after TAVI group, likely due to perceived higher chances of straightforward coronary re-access, as well as the inclusion of a wide spectrum of previous-generation self-expanding devices in the other groups, might have affected relevant outcomes, particularly device success and early safety5–8.
On the other hand, the lowest rate of major vascular complications and major bleeding observed in the PCI before TAVI group (despite a greater proportion of DAPT, DAT or TAT) might be attributed to the widest use of radial approach, reflecting different logistics and potential concerns for coronary re-access after TAVI in the other groups. Furthermore, the need for a higher amount of contrast medium with consequent higher rates of acute kidney injury might explain the higher mortality observed in patients undergoing PCI concomitant with TAVI.
Finally, although a previous analysis of the REVASC-TAVI registry demonstrated no differences between complete on incomplete myocardial revascularization, it is not clear whether an interaction exists between PCI timing and extent of revascularization3.
Overall, the results of this study support performing PCI after TAVI in most cases after a thorough clinical evaluation, and refraining from simultaneous treatment of both AS and CAD in the same session. However, given the observational, retrospective design of the study and its limitations mainly represented by significantly unbalanced number of patients across the treatment groups, further randomized data are warranted to confirm these findings.
References
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sadaba JR, Tribouilloy C, Wojakowski W, Neumann FJ, Myers P, Abdelhamid M, Achenbach S, Asteggiano R, Barili F, Borger MA, Carrel T, Collet JP, Foldager D, Habib G, Hassager C, Irs A, Iung B, Jahangiri M, Katus HA, Koskinas KC, Massberg S, Mueller CE, Nielsen JC, Pibarot P, Rakisheva A, Roffi M, Rubboli A, Shlyakhto E, Siepe M, Sitges M, Sondergaard L, Sousa-Uva M, Tarantini G, Zamorano JL, Benchabi Y, Chilingaryan A, Metzler B, Rustamova Y, Shumavets V, Lancellotti P, Smajic E, Trendafilova-Lazarova D, Samardzic J, Karakyriou M, Palecek T, Dahl JS, Meshaal MS, Palm K, Virtanen M, Bouleti C, Bakhutashvili Z, Boutsikou M, Kertész AB, Danielsen R, Topilsky Y, Golino P, Tuleutayev R, Elezi S, Kerimkulova A, Rudzitis A, Glaveckaite S, Sow R, Demarco DC, Bulatovic N, Aouad A, Van Den Brink R, Antova E, Beitnes JO, Ochala A, Ribeiras R, Vinereanu D, Irtyuga O, Ivanovic B, Simkova I, Gomez AG, Sarno G, Pedrazzini GB, Bsata W, Zakhama L, Korkmaz L, Cherniuk S, Khanji MY, Sharipov I, Baigent C, Aboyans V, Antoniou S, Arbelo E, Baumbach A, Čelutkiene J, Cikes M, Falk V, Fauchier L, Gale CP, Halvorsen S, Jaarsma T, Konradi A, Kotecha D, Landmesser U, Lewis BS, Linhart A, Løchen ML, Neubeck L, Petersen SE, Prescott E, Touyz RM, Galletti L, Hazekamp M, Licht P, Perier P, Prager R, Roessner E, Tsagakis K, Zientara A. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632.
- Patterson T, Clayton T, Dodd M, Khawaja Z, Morice MC, Wilson K, Kim WK, Meneveau N, Hambrecht R, Byrne J, Carrié D, Fraser D, Roberts DH, Doshi SN, Zaman A, Banning AP, Eltchaninoff H, Le Breton H, Smith D, Cox I, Frank D, Gershlick A, de Belder M, Thomas M, Hildick-Smith D, Prendergast B, Redwood S, Pocock S, Wang D, Irague F. ACTIVATION (PercutAneous Coronary inTervention prIor to transcatheter aortic VAlve implantaTION): A Randomized Clinical Trial. JACC Cardiovasc Interv. 2021;14:1965–74.
- Costa G, Pilgrim T, Amat Santos IJ, De Backer O, Kim WK, Ribeiro HB, Saia F, Bunc M, Tchetche D, Garot P, Ribichini FL, Mylotte D, Burzotta F, Watanabe Y, De Marco F, Tesorio T, Rheude T, Tocci M, Franzone A, Valvo R, Savontaus M, Wienemann H, Porto I, Gandolfo C, Iadanza A, Bortone AS, Mach M, Latib A, Biasco L, Taramasso M, Zimarino M, Tomii D, Nuyens P, Sondergaard L, Camara SF, Palmerini T, Orzalkiewicz M, Steblovnik K, Degrelle B, Gautier A, Del Sole PA, Mainardi A, Pighi M, Lunardi M, Kawashima H, Criscione E, Cesario V, Biancari F, Zanin F, Joner M, Esposito G, Adam M, Grube E, Baldus S, De Marzo V, Piredda E, Cannata S, Iacovelli F, Andreas M, Frittitta V, Dipietro E, Reddavid C, Strazzieri O, Motta S, Angellotti D, Sgroi C, Kargoli F, Tamburino C, Barbanti M. Management of Myocardial Revascularization in Patients With Stable Coronary Artery Disease Undergoing Transcatheter Aortic Valve Implantation. Circ Cardiovasc Interv. 2022;15:E012417.
- Rheude T, Costa G, Ribichini FL, Pilgrim T, Amat Santos IJ, De Backer O, Kim W-K, Ribeiro HB, Saia F, Bunc M, Tchetche D, Garot P, Mylotte D, Burzotta F, Watanabe Y, Bedogni F, Tesorio T, Tocci M, Franzone A, Valvo R, Savontaus M, Wienemann H, Porto I, Gandolfo C, Iadanza A, Bortone AS, Mach M, Latib A, Biasco L, Taramasso M, Zimarino M, Tomii D, Nuyens P, Sondergaard L, Camara SF, Palmerini T, Orzalkiewicz M, Steblovnik K, Degrelle B, Gautier A, Del Sole PA, Mainardi A, Pighi M, Lunardi M, Kawashima H, Criscione E, Cesario V, Biancari F, Zanin F, Esposito G, Adam M, Grube E, Baldus S, De Marzo V, Piredda E, Cannata S, Iacovelli F, Andreas M, Frittitta V, Dipietro E, Reddavid C, Strazzieri O, Motta S, Angellotti D, Sgroi C, Xhepa E, Kargoli F, Tamburino C, Joner M, Barbanti M. Comparison of different percutaneous revascularisation timing strategies in patients undergoing transcatheter aortic valve implantation. EuroIntervention. 2023.
- Abdel-Wahab M, Mehilli J, Frerker C, Neumann FJ, Kurz T, Tölg R, Zachow D, Guerra E, Massberg S, Schaf̈er U, El-Mawardy M, Richardt G. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA. 2014;311:1503–14.
- Thiele H, Kurz T, Feistritzer HJ, Stachel G, Hartung P, Eitel I, Marquetand C, Nef H, Doerr O, Lauten A, Landmesser U, Abdel-Wahab M, Sandri M, Holzhey D, Borger M, Ince H, Öner A, Meyer-Saraei R, Wienbergen H, Fach A, Frey N, König IR, Vonthein R, Rückert Y, Funkat AK, De Waha-Thiele S, Desch S. Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: the randomized SOLVE-TAVI trial. Eur Heart J. 2020;41:1890–9.
- Deharo P, Bisson A, Herbert J, Lacour T, Etienne C Saint, Grammatico-Guillon L, Porto A, Collart F, Bourguignon T, Cuisset T, Fauchier L. Impact of Sapien 3 Balloon-Expandable Versus Evolut R Self-Expandable Transcatheter Aortic Valve Implantation in Patients With Aortic Stenosis: Data From a Nationwide Analysis. Circulation. 2020;141:260–8.
- Costa G, Saia F, Pilgrim T, Abdel-Wahab M, Garot P, Valvo R, Gandolfo C, Branca L, Latib A, Santos IA, Mylotte D, De Marco F, De Backer O, Franco LN, Akodad M, Mazzapicchi A, Tomii D, Laforgia P, Cannata S, Fiorina C, Scotti A, Lunardi M, Poletti E, Mazzucca M, Quagliana A, Hennessey B, Meier D, Adamo M, Sgroi C, Reddavid CM, Strazzieri O, Motta SC, Frittitta V, Dipietro E, Comis A, Melfa C, Thiele H, Webb JG, Søndergaard L, Tamburino C, Barbanti M. Transcatheter Aortic Valve Replacement With the Latest-Iteration Self-Expanding or Balloon-Expandable Valves: The Multicenter OPERA-TAVI Registry. JACC Cardiovasc Interv. 2022;15:2398–407.







No comments yet!