22 Nov 2023
Electrosurgical laceration and stabilisation of three clip devices (ELASTA-Clip) to enable transcatheter mitral valve implantation
Selected in EuroIntervention Journal by M. Alasnag
ELASTA-Clip is a novel technique that offers a potential solution for individuals in whom the mitral regurgitation worsens or recurs.
References
Authors
Jonathan Curio, Elmar W. Kuhn, Maria Isabel Körber, Stephan Baldus, Jaffar M. Khan, Matti Adam
Reference
EuroIntervention 2023;19:744-745. DOI: 10.4244/EIJ-D-23-00596
Published
November 17, 2023
Link
Read the abstractReviewer
My Comment
Why this study – the rationale/objective?
Mitral transcatheter edge to edge repair (MTEER) has become a guideline recommended therapy offered to high-risk patients who are not suitable for surgical repair or replacement1. Its role in complex anatomies is under investigation and early reports of successful MTEER in mitral annulus calcification, for example, have been published2.
However, a limitation of MTEER is that it prohibits future transcatheter replacement in individuals in whom the mitral regurgitation (MR) worsens or recurs. The rate of recurrent MR is reported to be 9.8 % at 12-month follow-up after successful MitraClip, and even higher in some observational data3.
Electrosurgical Laceration and Stabilization of Clip devices (ELASTA-Clip) is a novel technique that offers a potential solution for these patients.
How was it executed – the methodology?
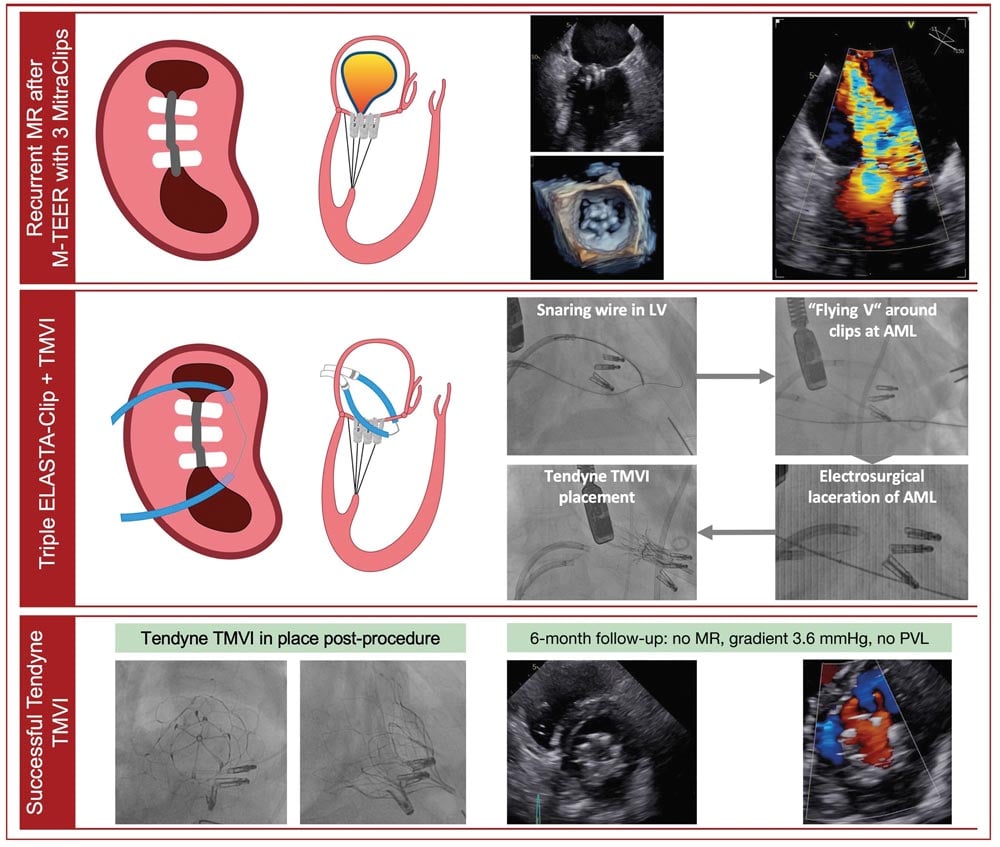
The technique employs electrocautery to lacerate and interrupt mitral leaflet tissue permitting the delivery & expansion of transcatheter mitral valve implants (TMVI). The technique requires transapical access and a 24 Fr DrySeal sheath in the femoral vein through which an Agilis NxT (Abbott Vascular) guiding sheaths traverse into the left atrium across the interatrial septum.
Similar to the Basilica of the aortic valve, an Astato 0.014 coronary guidewire (Asahi Intecc), which is both denuded and shaped into a sharp V, is used. This wire serves as the conduit to deliver the cautery current. Once the anterior leaflet is released, a TMV can be implanted.

Central illustration: ELASTA-Clip electrosurgical laceration of three MitraClip M-TEER devices and subsequent TMVI. AML: anterior mitral valve leaflet; LV: left ventricle; MR: mitral regurgitation; M-TEER: mitral transcatheter edge-to-edge repair; PVL: paravalvular leakage; TMVI: transcatheter mitral valve implantation
Source: EuroIntervention
Critical reading and the relevance for clinical practice
The technique is interesting; however, the learning curve remains steep, particularly since the volume of cases remains low currently. Perhaps referral centers where the expertise can be concentrated and developed is a necessity.
In addition, a dedicated device would simplify the procedure and likely reduce cost. At this time, each item is an additional cost to the procedure.
As the evidence for TMVI grows4, the need for the ELASTA technique will likely increase. MTEER patients are now offered better medical therapy, rehabilitation and follow-up and hence their survival rates have also increased in recent years.
Another important consideration is that younger patients undergoing MTEER have better survival and quality of life as well as lower heart failure rehospitalizations5. As such, the lifetime management of patients with severe MR must be addressed upfront especially if a transcatheter strategy is considered.
Yet, I wonder whether as the TMVI technology is further refined with transseptal solutions, would it be more reasonable to proceed with transcatheter replacement as a more durable and definitive therapy instead of MTEER? Will MTEER disappear in the near future?
References
- Correction to: 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2023 Nov 14;148(20):e185. doi: 10.1161/CIR.0000000000001190. Epub 2023 Nov 13. Erratum for: Circulation. 2021 Feb 2;143(5):e72-e227.
- Hatab T, Bou Chaaya RG, Zaid S, Wessly P, Satish P, Villanueva V, Faza N, Little SH, Atkins MD, Reardon MJ, Kleiman NS, Zoghbi WA, Goel SS. Feasibility and Outcomes of Mitral Transcatheter Edge-To-Edge Repair in Patients With Variable Degrees of Mitral Annular Calcification. J Am Heart Assoc. 2023 Oct 3;12(19):e031118. doi: 10.1161/JAHA.123.031118.
- Sugiura A, Kavsur R, Spieker M, Iliadis C, Goto T, Öztürk C, Weber M, Tabata N, Zimmer S, Sinning JM, Mauri V, Horn P, Kelm M, Baldus S, Nickenig G, Westenfeld R, Pfister R, Becher MU. Recurrent Mitral Regurgitation After MitraClip: Predictive Factors, Morphology, and Clinical Implication. Circ Cardiovasc Interv. 2022 Mar;15(3):e010895. doi: 10.1161/CIRCINTERVENTIONS.121.010895.
- Ludwig S, Conradi L, Cohen DJ, Coisne A, Scotti A, Abraham WT, Ben Ali W, Zhou Z, Li Y, Kar S, Duncan A, Lim DS, Adamo M, Redfors B, Muller DWM, Webb JG, Petronio AS, Ruge H, Nickenig G, Sondergaard L, Adam M, Regazzoli D, Garatti A, Schmidt T, Andreas M, Dahle G, Walther T, Kempfert J, Tang GHL, Redwood S, Taramasso M, Praz F, Fam N, Dumonteil N, Obadia JF, von Bardeleben RS, Rudolph TK, Reardon MJ, Metra M, Denti P, Mack MJ, Hausleiter J, Asch FM, Latib A, Lindenfeld J, Modine T, Stone GW, Granada JF; CHOICE-MI and the COAPT Trial Investigators. Transcatheter Mitral Valve Replacement Versus Medical Therapy for Secondary Mitral Regurgitation: A Propensity Score-Matched Comparison. Circ Cardiovasc Interv. 2023 Jun;16(6):e013045. doi: 10.1161/CIRCINTERVENTIONS.123.013045.
- Song C, Madhavan MV, Lindenfeld J, Abraham WT, Kar S, Lim DS, Grayburn PA, Kapadia SR, Kotinkaduwa LN, Mack MJ, Stone GW. Age-Related Outcomes After Transcatheter Mitral Valve Repair in Patients With Heart Failure: Analysis From COAPT. JACC Cardiovasc Interv. 2022 Feb 28;15(4):397-407. doi: 10.1016/j.jcin.2021.11.037.