27 Oct 2025
A randomised, multicenter, non-inferiority comparison of intravascular lithotripsy and super-high-pressure non-compliant balloons for treatment of calcified and refractory coronary lesions - The VICTORY trial
Reported from TCT 2025
Mirvat Alasnag provides her take on the VICTORY trial presented by Matthias Bossard at TCT 2025 in San Francisco.
Designed by: Mirvat Alasnag
Source: PCRonline.com
Background
Calcified lesions remain a challenging interventional procedure. Stent delivery and expansion are overcome with plaque modifying tools such as atherectomy devices and, more recently, intravascular lithotripsy.
The event rates, however, are higher than simpler lesions. There is little randomised data demonstrating superiortiy of modern devices over conventional tools. For example, the ECLIPSE trial noted the safety of non-complaint balloons (NCB) compated with orbital atherectomy. The FDA approval trials for intravascular lithotripsy (IVL) were single arm with no control group.
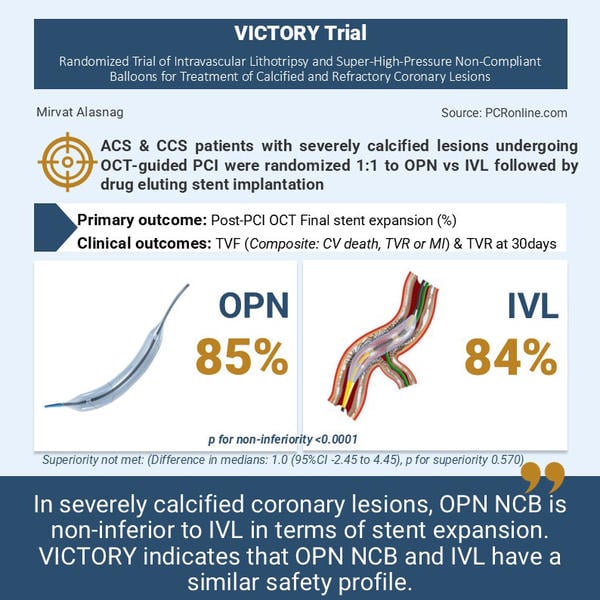
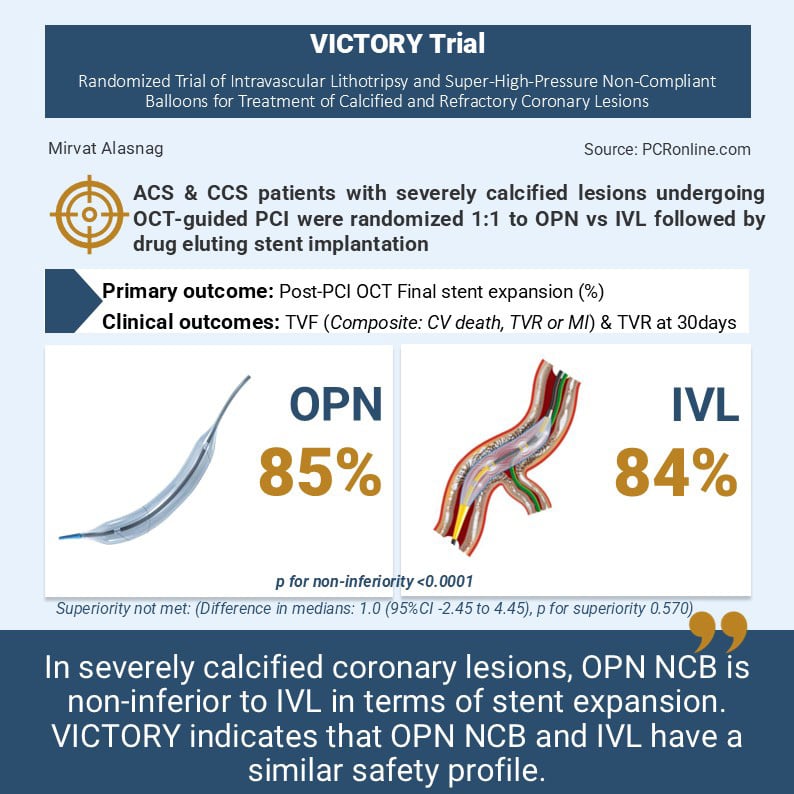
As such, the VICTORY trial sought to assess whether lesion preparation using the super high-pressure OPN NCB is non-inferior to a strategy involving IVL, in terms of the completeness of final stent expansion (SE), measured as a percentage (%) by optical coherence tomography (OCT) in patients with heavily calcified coronary lesions. The study also examined the safety of a strategy of OPN NCB compared with IVL for the treatment of heavily calcified lesions treated with drug eluting stents.
Methodology
The trialists assumed a non-inferiority margin of 10 % of stent expansion and estimated that with 280 patients – 140 patients assigned to each study arm – the study will have a 90 % power to demonstrate the non-inferiority with a one-sided alpha of 0.025. Patients were included if they fulfilled the following criteria:
Clinical inclusion cirteria | Angiographic inclusion criteria |
Age ≥ 18 years and eligible to consent | Single de novo target lesion stenosis of protected LMCA, or LAD, RCA or LCX (or of their branches) with:
|
Acute or chronic coronary artery disease with ischemia related symptoms (e.g. angina) and/or evidence of myocardial ischemia (e.g. FFR/ iFR, CMR, SPECT or PET-CT) | Plus at least one of the following criteria:
|
The exclusion criteria were acute ST elevation myocardial infarction (AMI) or cardiogenic shock related to an AMI, anatomy where the device or OCT catheter are unlikely to be delivered due to tortuosity or other characteristics, target lesion is in a coronary artery bypass graft and flow limiting target vessel thrombus.
Patients were randomised 1:1 to OPN or IVL strategy followed by implantation of a drug eluting stent. The primary outcome was an OCT confirmed stent expansion. The safety endpoint clinical follow up at 30 days with anticipated follow-up extending to 1-2 years. The secondary outcomes included acceptable stent expansion (> 80 %) and optimal stent expansion (> 90 %) assessed by OCT, acute procedural success, target vessel failure defined as cardiac death, target vessel MI or target vessel revascularisation.
Results
A total of 278 patients were enrolled, with a mean age of 71 years, of whom women were 12-18 % only. 54-59 % presented with a chronic coronary syndrome.
The proximal left anterior descending artery (LAD) was the target lesion in 40 % of those enrolled. Core lab adjudication noted that severe calcification was detected angiographically in 40 %, and by OCT in 43-45 % with nodular calcification in 24-27 %. The procedure time was numerically higher in the IVL arm (mean 79 vs 70 minutes; P = 0.06). The use of NCB prior to the study device was significantly higher in the IVL arm (40 % vs 23 %) although the use of atherectomy devices and scoring balloons was similar. The number of devices used was also significantly higher in the IVL arm.
The primary outcome was final stent expansion measured by OCT (85.0 % with OPN and 84.0 % with IVL). OPN NCB did not demonstrate superiority over IVL for the primary outcome (difference in medians: 1.0 (95 % CI -2.45 to 4.45), p for superiority 0.570). However, it did achieve its non-inferiority margin with a p for non-inferiority < 0.0001. These results were consistent across subgroups. In terms of the safety and secondary endpoints, there were no differences between the groups. There were no differences in 30-day outcomes such as periprocedural MI, target vessel and target lesion revascularisation, stent thrombosis, death, or bypass surgery.
Commentary
The trial was not powered for clinically relevant outcomes with stent expansion by OCT serving more as a surrogate parameter. Importantly, OPN NCB and IVL size selection was determined by OCT measurement, which is not as widely used as angiography or intravascular ultrasound, thereby limiting its generalisability.
The trial was conducted in 2 centers only with expert operators. Once again, their results in terms of safety and even stent expansion may not be generalisable to the global community.
The number of devices reported in the trial is an indication of the commonly used armamentarium in current practice where NCB complement IVL and these devices, including cutting balloons, are rarely standalone therapies. This particular endpoint, therefore, remains technically irrelevant.
What is relevant, however, is the cost effectiveness of the multiple devices used. As combination therapies and intracoronary imaging become mainstream practices, the cost will become a central conversation in every laboratory. Given the small number of patients enrolled, short follow-up and lack of power for clinical endpoints, the results of the trial will not change practice or guidelines at this time. However, they do demonstrate the safety of these modern tools for the treatment of calcified coronary lesions.




1 comment
Nice comments, however i do not think that the use of OPNC or IVL requires special experience as one can easily size with IVUS, furthermore i do not think that stent expansion demonstrated with OCT would be different than IVUS. However, the long term data are needed as we are not sure if OPNC cracls Calcium as well as IVL and therefore would lead to lumen loss with time