12 Jul 2023
Impact of alirocumab on plaque regression and haemodynamics of non-culprit arteries in patients with acute myocardial infarction: a prespecified substudy of the PACMAN-AMI trial
Selected in EuroIntervention by M. Alasnag , J. AlRahimi
This is a trial aiming to assess QFR and 3D-QCA DS% of all the non-infarct related arteries (IRA) with 3D-QCA DS% in all patients presenting with STEMI/NSTEMI and undergoing coronary angiography.
References
Authors
Sarah Bär; Raminta Kavaliauskaite; Tatsuhiko Otsuka; Yasushi Ueki; Jonas D. Häner; George C.M. Siontis; Stefan Stortecky; Hiroki Shibutani; Fabrice Temperli; Christoph Kaiser; Juan F Iglesias; Robert Jan van Geuns; Joost Daemen; David Spirk; Thomas Engstrøm; Irene Lang; Stephan Windecker; Konstantinos C. Koskinas; Sylvain Losdat; Lorenz Räber
Reference
EuroIntervention 2023;19:e-e. DOI: 10.4244/EIJ-D-23-00201
Published
21 June 2023
Link
Read the abstract
Reviewers
Our Comment
Why this study – the rationale/objective?
The World Heart Federation has indicated that targeting the accumulation of cholesterol-containing atherogenic lipoproteins in the vessel wall is a key strategy for the prevention of atherosclerotic cardiovascular disease (ASCVD) events. Historically, cardiovascular computed tomography studies have demonstrated that statin therapy resulted in significantly lower progression of low attenuation and non-calcified plaques1.
The SATURN study (Study of coronary Atheroma by inTravascular Ultrasound: the effect of Rosuvastatin vs. atorvastatiN) reported significant stablization in the indices of atheroma composition and regression of coronary atheroma itself on serial intravascular ultrasound (IVUS) in patients treated with rosuvastatin 40 mg or atorvastatin 80 mg daily for 24 months2. There is also mounting evidence suggesting that lipid-lowering therapy with statins is associated with an improvement in Quantitative flow ratio (QFR) and regression in two-dimensional quantitative coronary analysis diameter stenosis (2D)-QCA DS%.
As the armamentarium for the management of patients with hyperlipidemia continues to expand, the effects of PCSK9 inhibitors on QFR and 3D-QCA DS% and intracoronary parameters should be investigated. The PACMAN-AMI trial randomised, double-blind, placebo-controlled, European multicentre study was designed to evaluate the effect of intensive lipid-lowering therapy with alirocumab added to high-intensity statin therapy with rosuvastatin on coronary atherosclerosis defined by multimodality intracoronary imaging. The study itself demonstrated that compared with placebo, administration of alirocumab 150 mg bi-weekly within 24 hours after PCI for AMI results in a greater reduction in plaque burden and plaque regression at 52 weeks in the non-culprit vessel3.
How was it executed? - the methodology
This is a pre-specified sub-study of the PACMAN-AMI trial aiming to assess QFR and 3D-QCA DS% of all the non-infarct related arteries (IRA) with 3D-QCA DS% in all patients presenting with STEMI/NSTEMI (ST elevation myocardial infarction) and undergoing coronary angiography.
QFR and 3D-QCA were assessed at baseline and 1 year in any non-IRA ≥ 2.0 mm and 3D-QCA DS% > 25 %. The pre-specified primary endpoint was the number of patients with a mean QFR increase at 1 year, and the secondary endpoint was the change in 3D-QCA DS%.
Standard inclusion and exclusion criteria were employed:
Inclusion Criteria:
- 2 non-IRA suitable for intracoronary imaging with non-obstructive atherosclerotic disease (visual estimate > 20 to < 50 % angiographic DS%)
- LDL-C levels ≥ 125 mg/dl, if patients were statin-naïve or had not been on a stable (≥ 4 weeks) statin regimen at the time of screening;
- LDL-C ≥ 70 mg/dl, if patients were on an unchanged statin treatment for ≥ 4 weeks prior to study enrolment
Exclusion criteria:
- Absence of 2 projections with angles ≥ 25° apart,
- Lack of isocentre calibration
- Substantial vessel overlap or vessel foreshortening, severe tortuosity, poor contrast, ostial left main or right coronary artery (RCA) stenosis, TIMI flow ≤ 2, tachycardia > 100/min, and atrial or ventricular arrhythmia
The study primary endpoints included the number of patients with a mean increase in QFR across non-IRA from baseline to follow-up. The secondary endpoints included the continuous change in DS% by 3D-QCA and the continuous change in QFR from baseline to follow-up.
What is the main result?
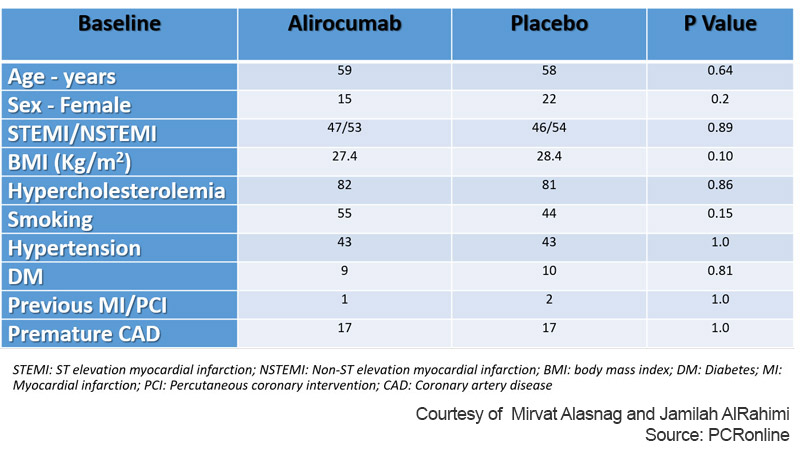
The study enrolled a total of 300 patients of whom 193 patients underwent serial QFR (94 Alirocumab group, 99 Placebo group). The total non-IRA evaluated were 282 (133 Alirocumab group, 149 Placebo group). The follow-up duration was 1 year. The mean patient age was 58 years and women were only 19 % of the total. Also, surprisingly, only 9 % had diabetes and the incidence of other risk factors was low (Table 1).
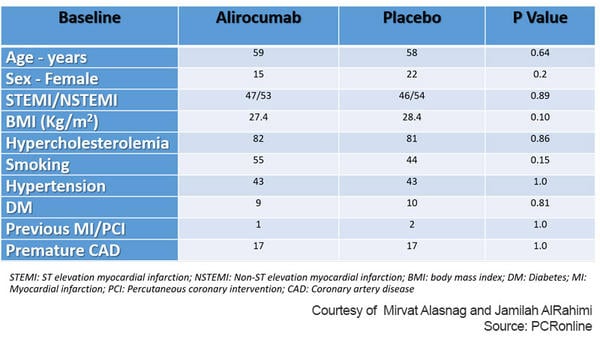
Table 1: Baseline characteristics of PACMAN AMI Substudy
Courtesy of Mirvat Alasnag and Jamilah AlRahimi
The baseline LDL level was 152.4 ± 33.7 mg/dl. The LDL-C levels decreased to 23.1 ± 21.0 mg/dl in the alirocumab arm compared to 78.3 ± 32.7 mg/dl in the placebo arm (p < 0.001) at one year. Although there were slightly more STEMI patients, there was no difference in the primary or secondary outcomes based on type of MI. The mean change in DS% was –1.03 ± 7.28 % in the alirocumab arm compared to +1.70 ± 8.27 % in the placebo arm (difference in change –2.50 %, 95 % CI: –4.43 to −0.57; p = 0.011).
Interestingly, authors note a trend towards more DS% reduction in vessels with a higher degree of baseline stenosis and an increase in the change in DS% between treatment groups favouring alirocumab. However, there no significant association between the change in DS% and intracoronary imaging variables.
Critical reading and the relevance for clinical practice
Overall, PCSK9 inhibitors did not result in a statistically significant increase in QFR compared to placebo. Alirocumab did, however, significantly improve coronary haemodynamics in flow-limiting disease. The sensitivity analysis excluding vessels with the no significant flow limitation (i.e., > 0.95) showed a significant improvement in the primary endpoint with alirocumab. This would suggest that lipid lowering is most useful in flow limiting disease; however, this is a very low risk population that was studied with a very low percentage of diabetes, premature ASCVD, other risk factors and previous MI or PCI. Whether ad difference would have been detected in non-obstructive coronary artery disease in a higher risk cohort remains speculative.
It is important to interpret the results of this trial in the context of the main PACMAN-AMI trial which conclusively reported plaque stabilisation with a 59 % increase in minimum fibrous cap thickness, a 30 % reduction in the fat content, and a 5 % relative reduction in plaque burden. The more accurate intracoronary imaging results are convincing and far less prone to technical factors affecting the accuracy and increasing the discordance particularly in the setting of an MI.
Aside from describing parameters that indicate plaque stabilization or regression, the more valuable evidence should be clinical outcomes (Figure 1). Both the Odyssey and Fourier trials have reported relevant cardiovascular outcomes in patients with ASCVD (including CAD and PAD). We wait the final results with the newer agents such as inclisiran in reducing major adverse cardiovascular events as well as reduction in plaque burden.
Figure 1: Goals of Lipid lowering therapy
Courtesy of Mirvat Alasnag and Jamilah AlRahimi
References
- Zeb I, Li D, Nasir K, Malpeso J, Batool A, Flores F, Dailing C, Karlsberg RP, Budoff M. Effect of statin treatment on coronary plaque progression - a serial coronary CT angiography study. Atherosclerosis. 2013 Dec;231(2):198-204. doi: 10.1016/j.atherosclerosis.2013.08.019. Epub 2013 Aug 29.
- Puri R, Libby P, Nissen SE, Wolski K, Ballantyne CM, Barter PJ, Chapman MJ, Erbel R, Raichlen JS, Uno K, Kataoka Y, Tuzcu EM, Nicholls SJ. Long-term effects of maximally intensive statin therapy on changes in coronary atheroma composition: insights from SATURN. Eur Heart J Cardiovasc Imaging. 2014 Apr;15(4):380-8. doi: 10.1093/ehjci/jet251. Epub 2014 Jan 20.
- Räber L, Ueki Y, Otsuka T, Losdat S, Häner JD, Lonborg J, Fahrni G, Iglesias JF, van Geuns RJ, Ondracek AS, Radu Juul Jensen MD, Zanchin C, Stortecky S, Spirk D, Siontis GCM, Saleh L, Matter CM, Daemen J, Mach F, Heg D, Windecker S, Engstrøm T, Lang IM, Koskinas KC; PACMAN-AMI collaborators. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients With Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA. 2022 May 10;327(18):1771-1781. doi: 10.1001/jama.2022.5218.
- Kirigaya H, Okada K, Hibi K, Maejima N, Iwahashi N, Matsuzawa Y, Akiyama E, Minamimoto Y, Kosuge M, Ebina T, Tamura K, Kimura K. Diagnostic performance and limitation of quantitative flow ratio for functional assessment of intermediate coronary stenosis. J Cardiol. 2021 May;77(5):492-499. doi: 10.1016/j.jjcc.2020.11.002. Epub 2020 Nov 24.