Effect of colchicine on coronary plaque stability in acute coronary syndrome as assessed by optical coherence tomography: the COLOCT randomized clinical trial
Selected in Circulation by G. Occhipinti , S. Brugaletta
The rationale for this study was to provide direct evidence of colchicine's effects on plaque stabilization, particularly by assessing changes in plaque characteristics using optical coherence tomography (OCT).
References
Authors
Miao Yu, Yong Yang, Si-Lai Dong, Chen Zhao, Fen Yang, Yuan-Fan Yuan, Yu-Hua Liao, Shao-Lin He, Kun Liu, Fen Wei, Hai-Bo Jia, Bo Yu, and Xiang Cheng
Reference
https://doi.org/10.1161/CIRCULATIONAHA.124.069808
Published
Originally Published 21 August 2024
Link
Read the abstractReviewers
Our Comment
Summary of the COLOCT trial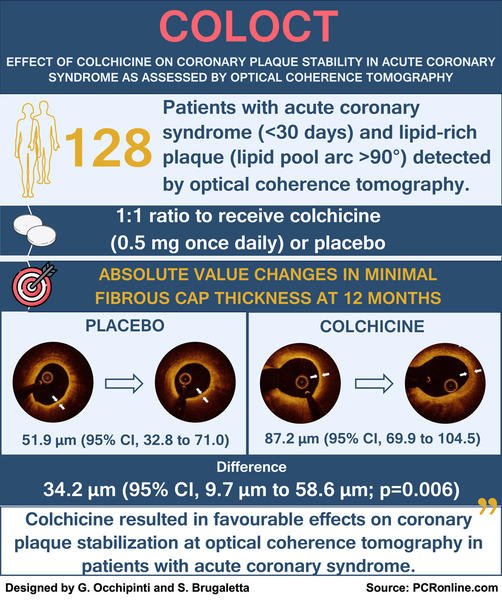
Designed by G. Occhipinti and S. Brugaletta - Source: PCRonline
Why this study – the rationale/objective?
Although colchicine, an anti-inflammatory drug, has been shown a signal toward reduced cardiovascular events in patients with coronary heart disease, its specific impact on coronary plaques has not been established.
The rationale for this study was to provide direct evidence of colchicine's effects on plaque stabilization, particularly by assessing changes in plaque characteristics using optical coherence tomography (OCT).
How was it executed – the methodology?
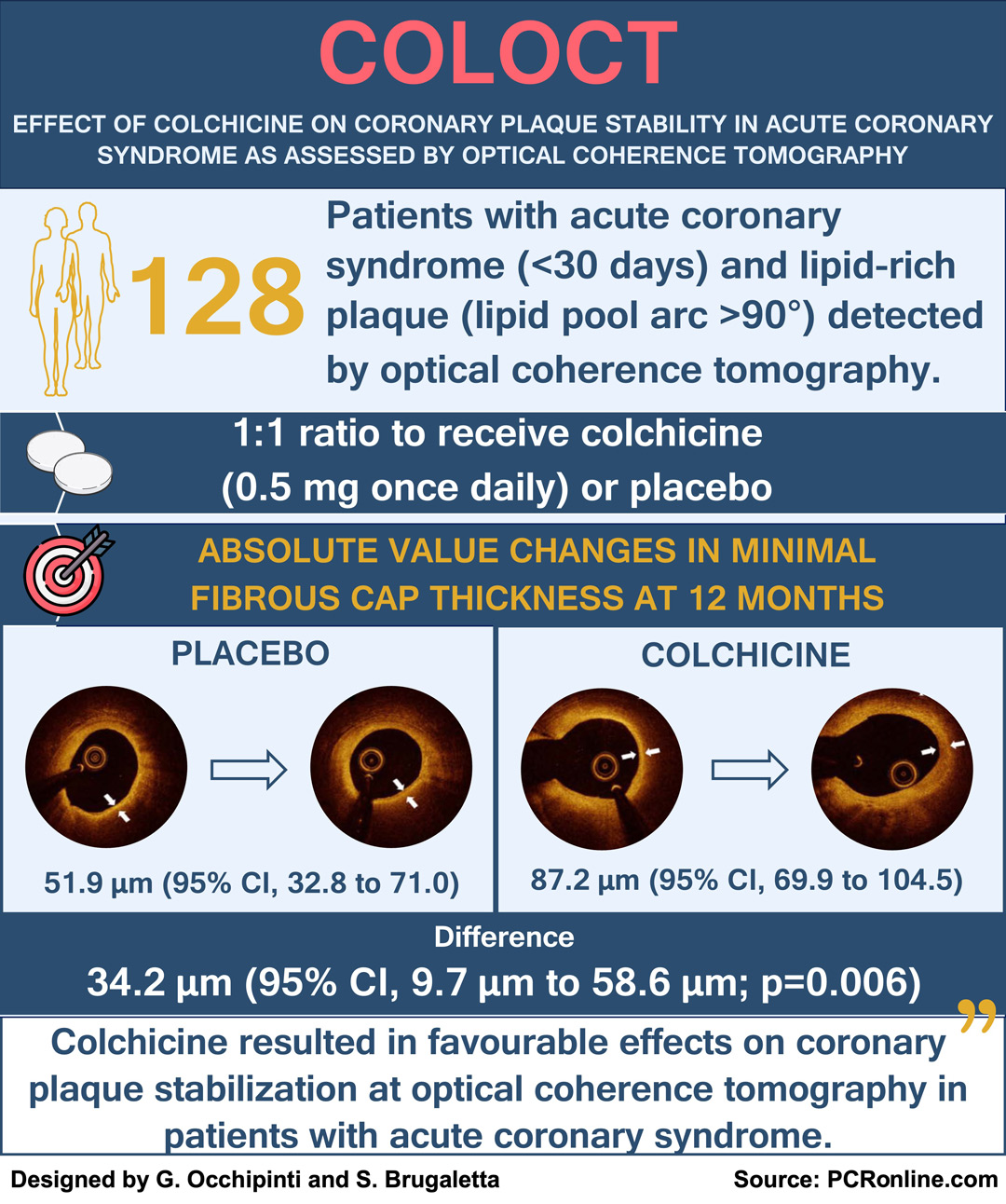
This was a single-center, randomized, double-blind, placebo-controlled clinical trial including 128 patients:
- diagnosed with acute coronary syndrome (ACS) within 1 month;
- with at least 1 non-culprit lesion of intermediate hemodynamic significance (30 to 70 % diameter stenosis) on coronary angiography,
- confirmed to be a lipid-rich plaque (lipid pool arc > 90°) by OCT1.
The subjects were randomly assigned in a 1:1 ratio to receive oral colchicine (0.5 mg once daily) or placebo for 12 months.
The primary endpoint was the change in the minimal fibrous cap thickness (FCT) from baseline to 12-month follow-up.
Secondary endpoints included:
- changes in average lipid arc;
- change in macrophage accumulation;
- change in incidence of thin-cap fibroatheroma (TCFA), defined as a lipid plaque with minimum FCT < 65 µm and lipid arc > 90° in circumference;
- and change in inflammatory biomarker levels [high-sensitivity-C reactive protein (hs-CRP), interleukin-6 (IL-6), and myeloperoxidase (MPO)];
- major adverse cardiovascular and cerebrovascular events.
What is the main result?
Colchicine therapy significantly increased minimal FCT (between groups difference, 34.2 μm; 95 % CI, 9.7 μm to 58.6 μm; p = 0.006), reduced average lipid arc (between groups difference, –10.5°; 95 % CI, –17.7° to –3.4°; p = 0.004), and mean angular extension of macrophages (between groups difference, –6.0°; 95 % CI, –11.8° to –0.2°; p = 0.044), compared with placebo.
The absolute value change of minimal FCT in patients with baseline low-density lipoprotein cholesterol (LDL-C) ≤ 70 mg/dL was greater than that in patients with baseline LDL-C > 70mg/dL (pint = 0.027).
There was no significant difference in the incidence of TCFA between the two groups.
A significant reduction was reported for hs-CRP levels (difference, 0.5; 95 % CI, 0.3 to 1.0; p = 0.046), IL-6 levels (difference, 0.6; 95 % CI, 0.4 to 0.9; p = 0.025), and MPO levels (difference, 0.8; 95% CI, 0.6 to 1.0; p = 0047).
Finally, the clinical endpoint (a composite of all-cause death, nonfatal myocardial infarction, nonfatal stroke, and revascularization because of ischemia) occurred in 17.3 % of patients in the placebo group vs 11.5 % in the colchicine group (p = 0.402).
Critical reading and the relevance for clinical practice
Evidence supporting the benefits of colchicine in stable ACS patients primarily comes from the COLCOT trial, which demonstrated a reduction in ischemic events in those treated with colchicine compared to placebo, with a significant reduction in the need for myocardial revascularization2.
In this context, although anti-inflammatory effects of colchicine are well established, the reasons behind these positive effects are still matter of debate3,4.
This study is the first demonstrating a positive impact of colchicine on coronary plaque stability using intravascular imaging. Colchicine, in particular, significantly increased minimal FCT. It is of note that these changes were primarily observed in not-overtly vulnerable plaques, characterized by a minimum lumen area of approximately 5 mm² and an initial FCT of 135 μm.
Additionally, only about 20 % of patients presented with thin-cap fibroatheromas, and among these, there was no significant difference in the incidence of TCFA after 12 months. Therefore, while the increase in FCT suggests a stabilizing plaque effect provided by colchicine, the clinical significance of these changes in terms of long-term outcomes remains to be determined.
It is also important to consider that 17.3 % of patients in the control group experienced ischemic events within one year, despite acceptable cholesterol levels (61.8 ± 23.2 mg/dL) and optimal medical therapy, highlighting an important unmet need in managing optimally the so-called residual cardiovascular risk5.
While it is too early to consider colchicine as a novel pharmacological approach to target vulnerable plaques, these results further support the potential of anti-inflammatory therapies for managing residual cardiovascular risk.
References
- Yu M, Yang Y, Dong SL et al. Effect of Colchicine on Coronary Plaque Stability in Acute Coronary Syndrome as Assessed by Optical Coherence Tomography: The COLOCT Randomized Clinical Trial. Circulation 2024.
- Tardif JC, Kouz S, Waters DD et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med 2019;381:2497-2505.
- Vaidya K, Arnott C, Martinez GJ et al. Colchicine Therapy and Plaque Stabilization in Patients With Acute Coronary Syndrome: A CT Coronary Angiography Study. JACC Cardiovasc Imaging 2018;11:305-316.
- Buckley LF, Libby P. Colchicine's Role in Cardiovascular Disease Management. Arterioscler Thromb Vasc Biol 2024;44:1031-1041.
- Everett BM. Residual Inflammatory Risk: A Common and Important Risk Factor for Recurrent Cardiovascular Events. J Am Coll Cardiol 2019;73:2410-2412





No comments yet!