Intra-annular self-expanding or balloon-expandable TAVI in small annuli: the NAVULTRA registry
Selected in EuroIntervention Journal by A. McInerney
Patients with severe symptomatic aortic stenosis and small annuli—most often women—face higher risks of elevated gradients, patient–prosthesis mismatch and early valve failure after TAVI, with added challenges for future coronary access and redo procedures, particularly in younger populations.
While previous studies have mainly compared supra-annular balloon- and self-expanding valves, this NAVULTRA substudy focuses on two intra-annular platforms, evaluating Sapien 3 ULTRA versus Navitor in this high-risk cohort.
References
Authors
Stefano Cannata, Ibrahim Sultan, Nicolas M. Van Mieghem, Arturo Giordano, Ole De Backer, Jonathan Byrne, Didier Tchétché, Sergio Buccheri, Luis Nombela-Franco, Rui Campante Teles, Marco Barbanti, Emanuele Barbato, Ignacio Amat Santos, Daniel J. Blackman, Francesco Maisano, Roberto Lorusso, Ketty La Spina, Antonella Millin, Dustin E. Kliner, Mark van den Dorpel, Elena Acerbi, avorka Lulic, Hossam Fayed, Chiara De Biase, Jorge Francisco Chavez Solsol, Joao Brito, Giuliano Costa, Matteo Casenghi, Clara Fernandez Cordon, Amanda Sherwen, Nicola Buzzatti, Salvatore Pasta, Marco Turrisi, Paolo Manca, Vincenzo Nuzzi, Corrado Tamburino, Francesco Bedogni, Caterina Gandolfo, Azeem Latib
Reference
DOI: 10.4244/EIJ-D-25-00937 - 22, 3, pages 161-171
Published
Feb 2, 2026
Link
Read the abstractReviewer
My Comment
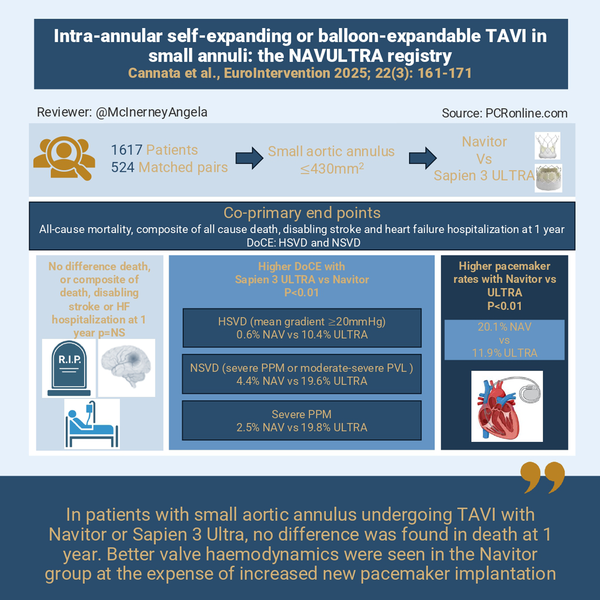
Infographic summing up the key points of the NAVULTRA REGISTRY proposed by Angela McInerney for PCRonline
Why this study – the rationale/objective?
Patients with symptomatic severe aortic stenosis and small aortic annuli present specific challenges when performing TAVI, a challenge that disproportionately affects women. Increased transvalvular gradients, patient prosthesis mismatch (PPM) and early bioprosthetic valve failure are well documented in this cohort. Furthermore, lifelong management including coronary re-access and TAV-in-TAV options are often limited in these patients, a consequence likely to become more prominent as TAVI is performed in younger populations.
Much debate has centred around the most appropriate index TAVI in these patients. Balloon expandable (BEV) and self-expanding (SEV) supra-annular devices have been compared in previous studies1-5. Comparison between intra-annular devices are less frequent. This substudy of the NAVULTRA registry therefore aimed to compare the intra-annular balloon expandable Sapien 3 Ultra (ULTRA) device to the intra-annular self-expanding Navitor (NAV) device in patients with small annuli6.
How was it executed? The methodology
This study compared two intra-annular devices: the balloon expandable Sapien 3 ULTRA and the self-expanding Navitor platform in patients with severe aortic stenosis and small aortic annuli undergoing TAVI6. Consecutive patients undergoing transfemoral TAVI with annular area ≤ 430 mm2 measured on pre-TAVI computed tomography were included. Main exclusions were TAVI in surgical prosthesis and those with incomplete CT follow up data. Groups were compared using 1:1 propensity score matching for the primary and secondary outcomes and adverse events were compared using Cox-proportional hazards method and Kaplan-Meier analysis.
A number of primary outcomes were examined:
- All-cause mortality
- A composite outcome including all-cause death, disabling stroke and heart failure hospitalisation at 1 year
- A composite device orientated end point (DoCE) of haemodynamic structural valve dysfunction (HSVD) (mean transvalvular gradient of ≥ 20 mmHg) and non-structural valve dysfunction (NSVD) (severe PPM or moderate-severe PVL).
Secondary outcomes were technical success, 30-day device success and 30-day early safety. All outcomes were defined using the VARC-3 criteria7.
What is the main result?
From 1,617 with small aortic annulus (787 NAV and 830 ULTRA), 524 matched pairs were obtained. Patients were predominantly female (> 75 %) with a mean age of 80.7 ± 6.7 years. Patients were of low-moderate surgical risk (mean STS-PROM 4.5 %) and the mean aortic annulus area was 377 ± 38 mm2.
Procedural characteristic differed with higher rates of pre- and post-dilation in the NAV group (predilation 79.2 % vs 21 %, postdilation 28.1 % vs 10.9 % for NAV and ULTRA respectively). Approximately 80 % of NAV patients received 25 or 27 mm valves, while ~80 % of ULTRA patients received 23 mm valves.
Regarding the primary outcomes there was no difference in all-cause mortality (8.8 vs 9.0 % for NAV and ULTRA respectively p = 0.449) or the composite end point of all-cause death, disabling stroke and heart failure hospitalisation (11.3 % vs 11.8 % for NAV and ULTRA respectively p = 0.463) at 1 year.
There was a significant difference in the DoCE:
Patients receiving NAV had reduced incidence of DoCE (6 % vs 29.3 % for NAV vs ULTRA p < 0.001) mainly driven by lower rates of HSVD and severe PPM in the NAV group (HSVD: 0.6 vs 10.4 % p < 0.01, severe PPM 2.5 % vs 18.8 %, p < 0.01 NAV vs ULTRA respectively).
Propensity score matched groups demonstrated similar results.
Similar rates of technical and device success were found however, early safety was higher in the ULTRA (75.6 % vs 82.6 % for NAV vs ULTRA respectively p < 0.01), mainly driven by increased new permanent pacemaker with NAV (new pacemaker at 30-days 18.2 % vs 10.4 %, p < 0.01, at 1 year 20.1 % vs 11.9 %, p < 0.01 for NAV vs ULTRA respectively). Mild PVL was also more frequently seen with NAV but no difference in ≥ moderate PVL was noted.
Critical reading and the relevance for clinical practice:
Device selection in patients with small aortic annuli requires balancing multiple factors including haemodynamic performance, risk of conduction disturbance and ease of future TAV-in-TAV options. This is particularly important in younger patients who are subject to the long-term consequences of these choices.
This substudy of the NAVULTRA study provides important insights into the performance of two intra-annular valve platforms in patients with small aortic annuli. No differences in the hard endpoints (all-cause mortality, disabling stroke and HF rehospitalisation) were found, however important differences in valve performance were noted which may influence valve choice with greater HSVD and PPM in the ULTRA group.
These results may not be surprising given that prior studies examining SEV versus BEV in this cohort demonstrated no differences in hard outcomes, such as death, with better haemodynamic outcomes for SEV’s at the price of higher rates of new pacemaker1-5. Most studies however examined the Evolut family compared to the Sapien family of valves. The NAV valve, being intra-annular may be expected to have higher gradients than the supra-annular Evolut; however the mean gradient for this platform was similar to that seen with the Evolut in the SMART trial (~7.4 mmHg). Similarly a substudy of the OCEAN-TAVI registry comparing Evolut and NAV platforms found no difference in mean transvalvular gradient or rates of PPM between these devices1,8. These data may suggest that, from a haemodynamic perspective at least, these devices could be considered interchangeable, and better than BEV.
The higher rate of DoCE in the ULTRA arm, driven by higher rates of HSVD and higher severe PPM may be expected when compared to recent trials. The SMART trial demonstrated moderate-severe PPM rates of 35 % with the ULTRA platform while other registries have ranged from 30-50 % reflecting rates in the current study2, 3, 9. Adverse consequences associated with PPM have been well documented in patients undergoing SAVR although debate surrounds its clinical significance in TAVI. A meta-analysis by Sá and colleagues, including 23 studies and ~ 81,000 patients demonstrated a higher risk of mortality in those with severe PPM after sensitivity analysis (HR: 1.25 [95 % CI: 1.16-1.36]; P < 0.001)10. Although the debate continues, avoiding moderate-severe PPM is desirable and based on studies to date, including the NAVULTRA study, more commonly achievable with self-expanding platforms.
Whether lower rates of PPM could be achieved with changes to implantation technique such as use of the “double tap” method, shown to improve expansion in ULTRA valves should be explored11. Low rates of pre- and post-dilation in the ULTRA arm of the NAVULTRA study are notable and may have contributed to PPM. The potential impact of universally utilising the ‘double tap’ technique on PPM rates with BEV in small annuli should encourage operators to take every care to optimise the index TAVI implant.
Finally, low pacemaker rates with BEV (~ 10 % or lower), even in small annuli have been found in previous studies and again in the NAVULTRA registry while that for the NAV platform neared 20 % within 30 days. SEV have been known to have higher pacemaker rates than BEV however these rates have been consistently decreasing due to both device iteration and changes to implantation technique12. The Evolut arm of the SMART trial had a new pacemaker rate of 12.1 %1. Implantation technique may have played a role in this higher rate given that the time period of the study spans 2018-2024 when deeper implants may have been accepted. More recent studies using the NAV device (albeit not in small annuli populations specifically) have attained new pacemaker rates < 10 %13. Nonetheless, this higher pacemaker rate is important for operators choosing devices, especially in younger patients with a long life expectancy, given the potential excess risk of adverse outcomes demonstrated in recent long term analysis14.
Other considerations in younger cohorts not specifically addressed in this study include coronary access and ease of redo TAV procedures which should factor into valve choice in this population.
Conclusion
Device choice in patients with small aortic annuli requires balancing the properties of each device with the specific needs of the patient. The NAVULTRA registry compares two intra-annular devices in this cohort: the self-expanding NAV and the balloon expandable Sapien 3 ULTRA. While no difference in mortality between TAVI platforms were found, there were important differences in haemodynamic performance and new pacemaker requirement. These results have potential implications particularly in younger patients where lifetime management aspects need to be considered and highlights the need for a tailored approach to device selection in patients with severe aortic stenosis and small aortic annuli.
References
- Herrmann HC, Mehran R, Blackman DJ, Bailey S, Mollmann H, Abdel-Wahab M, et al. Self-Expanding or Balloon-Expandable TAVR in Patients with a Small Aortic Annulus. N Engl J Med. 2024;390(21):1959-71.
- Scotti A, Sturla M, Costa G, Saia F, Pilgrim T, Abdel-Wahab M, et al. Evolut PRO and SAPIEN ULTRA Performance in Small Aortic Annuli: The OPERA-TAVI Registry. JACC Cardiovasc Interv. 2024;17(5):681-92.
- Okuno T, Tomii D, Lanz J, Heg D, Praz F, Stortecky S, et al. 5-Year Outcomes With Self-Expanding vs Balloon-Expandable Transcatheter Aortic Valve Replacement in Patients With Small Annuli. JACC Cardiovasc Interv. 2023;16(4):429-40.
- Leone PP, Regazzoli D, Pagnesi M, Cannata F, Mangieri A, Hokken TW, et al. Implantation of contemporary transcatheter aortic valves in small aortic annuli: the international multicentre TAVI-SMALL 2 registry. EuroIntervention. 2023;19(3):256-66.
- Hase H, Yoshijima N, Yanagisawa R, Tanaka M, Tsuruta H, Shimizu H, et al. Transcatheter aortic valve replacement with Evolut R versus Sapien 3 in Japanese patients with a small aortic annulus: The OCEAN-TAVI registry. Catheter Cardiovasc Interv. 2021;97(6):E875-E86.
- Cannata S, Sultan I, Van Mieghem NM, Giordano A, Backer O, Byrne J, et al. Intra-annular self-expanding or balloon-expandable TAVI in small annuli: the NAVULTRA registry. EuroIntervention. 2026;22(3):e161-e71.
- Varc-3 Writing C, Genereux P, Piazza N, Alu MC, Nazif T, Hahn RT, et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J Am Coll Cardiol. 2021;77(21):2717-46.
- Yamamoto M, Ryuzaki T, Hioki H, Kagase A, Shirai S, Ohno Y, et al. Intra- versus supra-annular self-expanding transcatheter heart valves in small aortic annuli. EuroIntervention. 2025;21(13):e749-e57.
- Tirado-Conte G, Rodes-Cabau J, Oteo JF, Pan M, Munoz E, Witberg G, et al. Transcatheter aortic valve implantation in patients with extra-small aortic annuli. EuroIntervention. 2023;19(4):e340-e51.
- Sa MP, Jacquemyn X, Van den Eynde J, Tasoudis P, Dokollari A, Torregrossa G, et al. Impact of Prosthesis-Patient Mismatch After Transcatheter Aortic Valve Replacement: Meta-Analysis of Kaplan-Meier-Derived Individual Patient Data. JACC Cardiovasc Imaging. 2023;16(3):298-310.
- Husain A, Jelisejevas J, Millar K, Alnuwakhtha A, Ferkh A, Delarive J, et al. Routine post-dilatation at nominal volume to optimise the expansion of balloon-expandable valves: the DOUBLE-TAP study. EuroIntervention. 2025;21(19):e1159-e68.
- Grubb KJ, Gada H, Mittal S, Nazif T, Rodes-Cabau J, Fraser DGW, et al. Clinical Impact of Standardized TAVR Technique and Care Pathway: Insights From the Optimize PRO Study. JACC Cardiovasc Interv. 2023;16(5):558-70.
- Worthley SG, Giordano A, Corcione N, Nombela-Franco L, De Marco F, Walton A, et al. 30-Day and 1-Year Outcomes of Navitor Transcatheter Aortic Valve in Low- or Intermediate-Risk Patients. JACC Cardiovasc Interv. 2025;18(20):2517-27.
- Badertscher P, Stortecky S, Serban T, Knecht S, Heg D, Tueller D, et al. Long-Term Outcomes of Patients Requiring Pacemaker Implantation After Transcatheter Aortic Valve Replacement: The SwissTAVI Registry. JACC Cardiovasc Interv. 2025;18(9):1163-71.






No comments yet!